Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins - PubMed (original) (raw)
Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins
K Tachibana et al. J Cell Biol. 2000.
Abstract
We have found a new cell-cell adhesion system at cadherin-based cell-cell adherens junctions (AJs) consisting of at least nectin and l-afadin. Nectin is a Ca(2+)-independent homophilic immunoglobulin-like adhesion molecule, and l-afadin is an actin filament-binding protein that connects the cytoplasmic region of nectin to the actin cytoskeleton. Both the trans-interaction of nectin and the interaction of nectin with l-afadin are necessary for their colocalization with E-cadherin and catenins at AJs. Here, we examined the mechanism of interaction between these two cell-cell adhesion systems at AJs by the use of alpha-catenin-deficient F9 cell lines and cadherin-deficient L cell lines stably expressing their various components. We showed here that nectin and E-cadherin were colocalized through l-afadin and the COOH-terminal half of alpha-catenin at AJs. Nectin trans-interacted independently of E-cadherin, and the complex of E-cadherin and alpha- and beta-catenins was recruited to nectin-based cell-cell adhesion sites through l-afadin without the trans-interaction of E-cadherin. Our results indicate that nectin and cadherin interact through their cytoplasmic domain-associated proteins and suggest that these two cell-cell adhesion systems cooperatively organize cell-cell AJs.
Figures
Figure 1
Structures of various constructs of nectin, l-afadin, ponsin, E-cadherin, and α-catenin. CTM, COOH-terminal motif of four aa residues; GAL4AD, GAL4 activation domain; and CBD, catenin-binding domain.
Figure 2
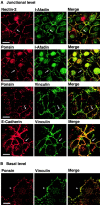
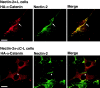
α-Catenin–dependent colocalization of nectin and l-afadin with E-cadherin. αF9Dα(−/−) and F9Dα(−/−) cells were double stained with various combinations of the anti–E-cadherin, anti–l-afadin, and anti–nectin-2 Abs. (arrows) Cell–cell adhesion sites between two cells; and (arrowheads) cell–cell adhesion sites where >2 cells adhere to each other. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 3
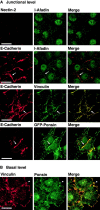
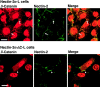
β-Catenin–independent colocalization of nectin, l-afadin, ponsin, and vinculin with E-cadherin. nEα-L cells were double stained with various combinations of the anti–nectin-2, anti–l-afadin, antiponsin, antivinculin, and anti–E-cadherin Abs. There was nuclear staining with the anti–l-afadin or antiponsin Ab, but the nuclear staining was not an artifact of second antibodies, although its significance is not known. (arrows) Cell–cell adhesion sites; and (arrowhead) focal contacts. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 4
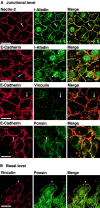
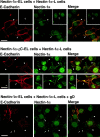
Nectin- and l-afadin–independent colocalization of ponsin and vinculin with E-cadherin. nEαN-L cells were double stained with various combinations of the anti–nectin-2, anti–l-afadin, antiponsin, antivinculin, and anti–E-cadherin Abs. Alternatively, nEαN-L cells were transfected with pEGFP-ponsin and stained with the anti–E-cadherin Ab. (arrows) Cell–cell adhesion sites; and (arrowhead) focal contacts. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 5
Ponsin- and vinculin-independent colocalization of nectin and l-afadin with E-cadherin. nEαC-L cells were double stained with various combinations of the anti–nectin-2, anti–l-afadin, antiponsin, antivinculin, and anti–E-cadherin Abs. (arrows) Cell–cell adhesion sites; and (arrowhead) focal contacts. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 6
Interaction of l-afadin with α-catenin on affinity chromatography. GST-α-catenin-C was applied to metal affinity beads on which Myc-His6-l-afadin was immobilized. After the beads were extensively washed, elution was performed with 100 mM imidazole chloride, pH 7.5. Each fraction was subjected to SDS-PAGE (10% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue. The results shown are representative of three independent experiments.
Figure 7
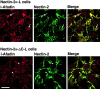
Localization of nectin and l-afadin in nectin-2α-L and -2α-ΔC-L cells. Nectin-2α-L and -2α-ΔC-L cells were double stained with the anti–nectin-2 and anti–l-afadin Abs. (arrows) Nectin-2α–based cell–cell adhesion sites; and (arrowheads) nectin-2α-ΔC–based cell–cell adhesion sites. The results shown are representative of three independent experiments. Bar, 10 μm.
Figure 8
l-Afadin–dependent recruitment of α-catenin to nectin-based cell–cell adhesion sites in the absence of E-cadherin. Nectin-2α-L and -2α-ΔC-L cells were transfected with pPGKIZ-HA-α-catenin and double stained with the anti–nectin-2 and anti-HA Abs. (arrow) Nectin-2α–based cell–cell adhesion sites; and (arrowhead) nectin-2α-ΔC–based cell–cell adhesion sites. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 9
l-Afadin–dependent recruitment of β-catenin to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin. Nectin-2α-L and -2α-ΔC-L cells were transfected with pEF-tEC and double stained with the anti–nectin-2 and anti–β-catenin Abs. (arrows) Nectin-2α–based cell–cell adhesion sites; and (arrowhead) nectin-2α-ΔC–based cell–cell adhesion sites. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 10
l-Afadin–dependent recruitment of E-cadherin to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin. Nectin-1α-L cells were cocultured with nectin-1α-EL or -1α-ΔC-EL cells in the presence or absence of gD and double stained with the anti–nectin-1α Ab1 and the anti–E-cadherin Ab. In the absence of gD, E-cadherin was concentrated at ∼40% of the adhesion sites between nectin-1α-L and -1α-EL cells. The recruitment of E-cadherin was observed in ∼20% of the adhesion sites between nectin-1α-L and -1α-ΔC-EL cells. In the presence of gD, the recruitment of E-cadherin was observed in ∼8% of the adhesion sites between nectin-1α-L and -1α-EL cells. (arrows) Cell–cell adhesion sites between two nectin-1α-L cells or between two nectin-1α-ΔC-L cells; (arrowheads) cell–cell adhesion sites between nectin-1α-L and -1α-EL or -1α-ΔC-EL cells; and (double arrowheads) cell–cell adhesion sites between two nectin-1α-EL cells or between two nectin-1α-ΔC-EL cells. The results shown are representative of three independent experiments.
Similar articles
- alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin.
Yokoyama S, Tachibana K, Nakanishi H, Yamamoto Y, Irie K, Mandai K, Nagafuchi A, Monden M, Takai Y. Yokoyama S, et al. Mol Biol Cell. 2001 Jun;12(6):1595-609. doi: 10.1091/mbc.12.6.1595. Mol Biol Cell. 2001. PMID: 11408571 Free PMC article. - Involvement of LMO7 in the association of two cell-cell adhesion molecules, nectin and E-cadherin, through afadin and alpha-actinin in epithelial cells.
Ooshio T, Irie K, Morimoto K, Fukuhara A, Imai T, Takai Y. Ooshio T, et al. J Biol Chem. 2004 Jul 23;279(30):31365-73. doi: 10.1074/jbc.M401957200. Epub 2004 May 12. J Biol Chem. 2004. PMID: 15140894 - Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells.
Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Sato T, et al. J Biol Chem. 2006 Feb 24;281(8):5288-99. doi: 10.1074/jbc.M510070200. Epub 2005 Dec 18. J Biol Chem. 2006. PMID: 16361708 - Nectin and afadin: novel organizers of intercellular junctions.
Takai Y, Nakanishi H. Takai Y, et al. J Cell Sci. 2003 Jan 1;116(Pt 1):17-27. doi: 10.1242/jcs.00167. J Cell Sci. 2003. PMID: 12456712 Review. - [Role of the nectin-afadin system in organization of tight and adherens junctions in epithelial cells].
Katata T, Irie K, Takai Y. Katata T, et al. Tanpakushitsu Kakusan Koso. 2003 Feb;48(2):105-12. Tanpakushitsu Kakusan Koso. 2003. PMID: 12638174 Review. Japanese. No abstract available.
Cited by
- Alternative molecular mechanisms for force transmission at adherens junctions via β-catenin-vinculin interaction.
Morales-Camilo N, Liu J, Ramírez MJ, Canales-Salgado P, Alegría JJ, Liu X, Ong HT, Barrera NP, Fierro A, Toyama Y, Goult BT, Wang Y, Meng Y, Nishimura R, Fong-Ngern K, Low CSL, Kanchanawong P, Yan J, Ravasio A, Bertocchi C. Morales-Camilo N, et al. Nat Commun. 2024 Jul 5;15(1):5608. doi: 10.1038/s41467-024-49850-5. Nat Commun. 2024. PMID: 38969637 Free PMC article. - The zonula adherens matura redefines the apical junction of intestinal epithelia.
Mangeol P, Massey-Harroche D, Sebbagh M, Richard F, Le Bivic A, Lenne PF. Mangeol P, et al. Proc Natl Acad Sci U S A. 2024 Feb 27;121(9):e2316722121. doi: 10.1073/pnas.2316722121. Epub 2024 Feb 20. Proc Natl Acad Sci U S A. 2024. PMID: 38377188 Free PMC article. - Adherens junctions as molecular regulators of emergent tissue mechanics.
Campàs O, Noordstra I, Yap AS. Campàs O, et al. Nat Rev Mol Cell Biol. 2024 Apr;25(4):252-269. doi: 10.1038/s41580-023-00688-7. Epub 2023 Dec 13. Nat Rev Mol Cell Biol. 2024. PMID: 38093099 Review. - SRGP-1/srGAP and AFD-1/afadin stabilize HMP-1/⍺-catenin at rosettes to seal internalization sites following gastrulation in C. elegans.
Serre JM, Slabodnick MM, Goldstein B, Hardin J. Serre JM, et al. PLoS Genet. 2023 Mar 3;19(3):e1010507. doi: 10.1371/journal.pgen.1010507. eCollection 2023 Mar. PLoS Genet. 2023. PMID: 36867663 Free PMC article. - Nectins and Nectin-like molecules drive vascular development and barrier function.
Hermans D, Rodriguez-Mogeda C, Kemps H, Bronckaers A, de Vries HE, Broux B. Hermans D, et al. Angiogenesis. 2023 Aug;26(3):349-362. doi: 10.1007/s10456-023-09871-y. Epub 2023 Mar 3. Angiogenesis. 2023. PMID: 36867287 Review.
References
- Adra C.N., Boer P.H., McBurney M.W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60:65–74. - PubMed
- Aoki J., Koike S., Ise I., Sato-Yoshida Y., Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J. Biol. Chem. 1994;269:8431–8438. - PubMed
- Aoki J., Koike S., Asou H., Ise I., Suwa H., Tanaka T., Miyasaka M., Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp. Cell Res. 1997;235:374–384. - PubMed
- Asakura T., Nakanishi H., Sakisaka T., Takahashi K., Mandai K., Nishimura M., Sasaki T., Takai Y. Similar and differential behavior between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells. 1999;4:573–581. - PubMed
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous