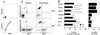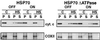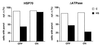The chaperone function of hsp70 is required for protection against stress-induced apoptosis - PubMed (original) (raw)
The chaperone function of hsp70 is required for protection against stress-induced apoptosis
D D Mosser et al. Mol Cell Biol. 2000 Oct.
Abstract
Cellular stress can trigger a process of self-destruction known as apoptosis. Cells can also respond to stress by adaptive changes that increase their ability to tolerate normally lethal conditions. Expression of the major heat-inducible protein hsp70 protects cells from heat-induced apoptosis. hsp70 has been reported to act in some situations upstream or downstream of caspase activation, and its protective effects have been said to be either dependent on or independent of its ability to inhibit JNK activation. Purified hsp70 has been shown to block procaspase processing in vitro but is unable to inhibit the activity of active caspase 3. Since some aspects of hsp70 function can occur in the absence of its chaperone activity, we examined whether hsp70 lacking its ATPase domain or the C-terminal EEVD sequence that is essential for peptide binding was required for the prevention of apoptosis. We generated stable cell lines with tetracycline-regulated expression of hsp70, hsc70, and chaperone-defective hsp70 mutants lacking the ATPase domain or the C-terminal EEVD sequence or containing AAAA in place of EEVD. Overexpression of hsp70 or hsc70 protected cells from heat shock-induced cell death by preventing the processing of procaspases 9 and 3. This required the chaperone function of hsp70 since hsp70 mutant proteins did not prevent procaspase processing or provide protection from apoptosis. JNK activation was inhibited by both hsp70 and hsc70 and by each of the hsp70 domain mutant proteins. The chaperoning activity of hsp70 is therefore not required for inhibition of JNK activation, and JNK inhibition was not sufficient for the prevention of apoptosis. Release of cytochrome c from mitochondria was inhibited in cells expressing full-length hsp70 but not in cells expressing the protein with ATPase deleted. Together with the recently identified ability of hsp70 to inhibit cytochrome c-mediated procaspase 9 processing in vitro, these data demonstrate that hsp70 can affect the apoptotic pathway at the levels of both cytochrome c release and initiator caspase activation and that the chaperone function of hsp70 is required for these effects.
Figures
FIG. 1
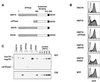
Stable cell lines with tetracycline-regulated expression of hsc70, hsp70, or hsp70 domain mutant proteins. (A) Schematic representation of hsp70 functional domains. hsp70 and hsc70 have an amino-terminal ATPase domain followed by a peptide binding domain and the C-terminal sequence EEVD. (B) Flow-cytometric profiles of GFP expression in each of the clones grown in the presence (shaded profile) and absence (open profile) of doxycycline. The expression cassette is dicistronic and carries both the gene of interest and the GFP gene. In the BFP-expressing cell line the dicistronic transcript carries both the BFP and GFP genes. All of the cell lines were induced for 24 h except for the hsc70 and BFP-expressing cell lines, which were induced for 48 h. (C) Western blot analysis showing the levels of expression of the proteins encoded by the first cistron. Cells were grown with or without doxycycline as described for panel B. The upper portion shows the results with antibody N27, which recognizes both hsc70 and hsp70. The bottom portion shows the results for hsp70-specific antibody C92. Purified hsp70 (25 to 400 ng) was included to insure linearity of the signals.
FIG. 2
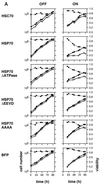
Effect of hsc70 and hsp70 expression on cell growth. (A) Time profiles of the accumulation of viable cells (open circles), total cells (solid circles), and cell viability (diamonds) for cells grown in the absence (OFF) or presence (ON) of doxycycline. (B) μapp and kd were calculated from the data shown in panel A, and the ratios of these values for the cells grown in the presence or absence of doxycycline were calculated. The calculated values for μapp ON and μapp OFF for cells expressing the indicated proteins are, respectively, as follows: hsc70 0.0176 and 0.0206 h−1; hsp70, 0.0057 and 0.0203 h−1; hsp70ΔATPase, 0.0084 and 0.0178 h−1; hsp70ΔEEVD, 0.0155 and 0.0229 h−1; hsp70AAAA, 0.0149 and 0.0222 h−1; BFP, 0.0172 and 0.0191 h−1. Corresponding calculated values for kd ON and kd OFF are, respectively, as follows: hsc70, 0.0071 and 0.0045 h−1; hsp70, 0.0161 and 0.0034 h−1; hsp70ΔATPase, 0.01 and 0.0041 h−1; hsp70ΔEEVD, 0.004 and 0.0022 h−1; hsp70AAAA, 0.0091 and 0.0031 h−1; BFP, 0.0044 and 0.0027 h−1.
FIG. 2
Effect of hsc70 and hsp70 expression on cell growth. (A) Time profiles of the accumulation of viable cells (open circles), total cells (solid circles), and cell viability (diamonds) for cells grown in the absence (OFF) or presence (ON) of doxycycline. (B) μapp and kd were calculated from the data shown in panel A, and the ratios of these values for the cells grown in the presence or absence of doxycycline were calculated. The calculated values for μapp ON and μapp OFF for cells expressing the indicated proteins are, respectively, as follows: hsc70 0.0176 and 0.0206 h−1; hsp70, 0.0057 and 0.0203 h−1; hsp70ΔATPase, 0.0084 and 0.0178 h−1; hsp70ΔEEVD, 0.0155 and 0.0229 h−1; hsp70AAAA, 0.0149 and 0.0222 h−1; BFP, 0.0172 and 0.0191 h−1. Corresponding calculated values for kd ON and kd OFF are, respectively, as follows: hsc70, 0.0071 and 0.0045 h−1; hsp70, 0.0161 and 0.0034 h−1; hsp70ΔATPase, 0.01 and 0.0041 h−1; hsp70ΔEEVD, 0.004 and 0.0022 h−1; hsp70AAAA, 0.0091 and 0.0031 h−1; BFP, 0.0044 and 0.0027 h−1.
FIG. 3
Protection from heat-induced apoptosis by hsc70 and hsp70 proteins. (A) Demonstration that GFP fluorescence intensity correlates with levels of hsp70. Noninduced (upper panel) and induced (lower panel) cells were fixed and processed for immunocytochemical detection of hsp70 using the hsp70-specific antibody C92 and a PE-conjugated antimouse antibody. (B) Analysis of cell viability by annexin-PE staining in the hsp70-expressing cell line. Control and heat-shocked (43°C for 60 min followed by 9 h at 37°C) cells were incubated with annexin-PE and then analyzed for PE and GFP fluorescence by flow cytometry. Quadrants 1 to 4 correspond to cells that are annexin+ GFP−, annexin+ GFP+, annexin− GFP−, and annexin− GFP+, respectively. (C) Results of annexin staining for each of the cell lines after heat shock. The left panel shows the means and standard errors (n = 3) for the noninduced (OFF) and induced (ON) cells. For the induced cells the viabilities were calculated for the GFP-positive cells only. The right panel shows the means plus standard errors of the differences between the viabilities of the ON and the OFF cells. Comparison of each of these means to that of the BFP-expressing cell line shows that only hsc70 and the intact hsp70 protein provide protection against apoptosis (*, P values of a one-tailed t test are 0.0002 for the HSC70 cell line, 0.001 for the HSP70 cell line, and 0.0008 for the PETA70 cell line; all other P values are greater than 0.05). The PETA70 cell line has tTA-regulated expression of hsp70.
FIG. 4
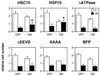
Cell viability after heat shock is enhanced by hsc70 and hsp70, and this requires the chaperone function of hsp70. Noninduced (OFF) and induced (ON) cells were heated at 43°C for 60 min and returned to 37°C for 24 h. Flow-cytometric cell counts were carried out before the heat shock and after the 24-h recovery period. Separate cultures of nonheated cells were also counted at the same times. Cell numbers at the end of the recovery period are plotted relative to the initial cell count for control (open bars) and heated cells (solid bars). Shown are the means plus the standard errors for three independent experiments. Only the cells expressing hsc70 or hsp70 have significantly higher numbers of viable cells after heat shock (*, P values of a one-tailed t test comparing the relative cell numbers after heat shock for the ON and OFF cells are 0.033 for the HSC70 cell line and 0.022 for the HSP70 cell line; all other P values are greater than 0.1).
FIG. 5
At equivalent levels of expression the full-length hsp70 protein provides protection whereas the protein with ATPase deleted does not. The HSP70 (clone 8-17) and HSP70ΔATPase cell lines were incubated for 24 h with various doses of doxycycline ([DOX]) ranging from 100 to 1,000 ng/ml and then examined for GFP fluorescence by flow cytometry (A), expressed protein levels by Western blotting (B), and resistance to heat-induced apoptosis (D) as described for Fig. 4. Quantitation of the Western blot (C) and comparison to the percent viabilities after heat shock (E) reveal that the full-length hsp70 protein protects cells from apoptosis when expressed at a level equal to the maximum level attained in the HSP70ΔATPase cell line and that the protein with ATPase deleted does not protect cells at any of the expressed levels.
FIG. 6
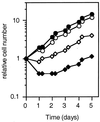
Cells protected from heat-induced apoptosis by hsp70 retain their proliferative capacity. The HSP70 cell line (clone 8-17), either noninduced (solid symbols) or incubated with 200 ng of doxycycline/ml for 24 h (open symbols), was exposed to 43°C for 60 min and then returned to 37°C (diamonds). The numbers of viable cells were determined, as described for Fig. 4, over a period of 5 days and are plotted relative to the initial cell count. The growth of control non-heat-shocked cells (circles) is shown for comparison.
FIG. 7
Inhibition of heat-induced JNK activation in hsc70- and hsp70-expressing cells. Western blot analysis showed levels of phosphorylated JNK1 and JNK2 in noninduced (OFF) and induced (ON) cells. Cells were either not treated (C), heated at 43°C for 60 min (HS), or heat shocked and returned to 37°C for 60 min (R). JNK activation was strongly inhibited in all of the induced cells lines except for the BFP-expressing cell line.
FIG. 8
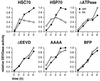
The C-terminal substitution mutant hsp70AAAA inhibits JNK activation without blocking its rate of dephosphorylation. IMR90 lung fibroblast cells (20 to 30 population doublings) were infected with an adenovirus expressing tTA (AdCMV-tTA) and an adenovirus encoding a tetracycline-regulated expression cassette encoding full-length hsp70 (+Hsp70) or hsp70 with the C-terminal four amino acids EEVD replaced with AAAA (+Hsp70-4AA). Control cells received the same total multiplicity of infection of the hsp70-encoding virus but without the Ad CMV-tTA virus (control). The infected cells were heat shocked for 30 min at 45°C and then incubated at 37°C with the protein kinase inhibitor staurosporine to block new JNK phosphorylation. Extracts were prepared from non-heat-shocked cells (Con.), heat-shocked cells (0), and heat-shocked cells that were incubated with staurosporine for 20 or 40 min at 37°C after the heat shock. Shown is an immunoblot of cytosolic extracts probed with an anti-phospho-JNK antibody.
FIG. 9
Inhibition of heat shock-induced caspase activation in cells expressing hsc70 or hsp70 but not in cells expressing the hsp70 deletion mutant proteins. Extracts were prepared from noninduced (solid circles) and induced (open circles) cells that were heated at 43°C for 60 min and collected either immediately after the heat shock (0) or after a return to 37°C for 3, 6, or 9 h. Extracts were also prepared from control non-heat-shocked cells (C). Caspase activity was measured using a fluorometric assay with the substrate Ac-DEVD-AMC. Activities (fluorescence units per minute per microgram of protein) are plotted relative to the maximum activity obtained for each cell line.
FIG. 10
Inhibition of procaspase 9 and procaspase 3 processing in cells expressing hsc70 or hsp70. Western blot analysis of extracts from noninduced (OFF) and induced (ON) control (C) and heat-shocked (HS; 43°C for 60 min followed by 6 h at 37°C) cells showing levels of the intact procaspase 9 protein, the intact procaspase 3 protein, and its processed large subunit (p17). Protection against loss of the proform of caspase 9 or 3 in the induced state occurs only for cells expressing hsc70 or hsp70.
FIG. 11
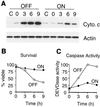
Release of cytochrome c from mitochondria after heat shock is blocked in hsp70-expressing cells. (A) Levels of cytochrome c and actin were measured by Western blotting of cytosolic extracts prepared by Dounce homogenization of noninduced (OFF) and induced (ON) HSP70 cells (clone 8-17). Extracts were prepared from nonstressed cells (C) and cells that were exposed to 43°C for 60 min and collected either immediately after the heat shock (0) or following incubation at 37°C for 3, 6, or 9 h. (B) Cell viability was measured at the time of cell collection by counting viable and apoptotic cells after staining with acridine orange and ethidium bromide. (C) Caspase activity in the extracts was measured as described for Fig. 9.
FIG. 12
Cytochrome c release from heat-shocked cells measured by streptolysin O permeabilization. Noninduced (OFF) and induced (ON) cells expressing hsp70 (clone 8-17) and hsp70ΔATPase were either not stressed (C) or heat shocked by exposure to 43°C for 60 min and then returned to 37°C for 6 h (HS). Cells were permeabilized by incubation with streptolysin O and then centrifuged to separate the cytosolic proteins (S) from the permeabilized cells (P). Equal volumes from each fraction were analyzed by Western blotting with antibodies to cytochrome c (cyt. c) and mitochondrial inner membrane protein cytochrome oxidase subunit II (COXII).
FIG. 13
Immunocytochemical examination of cytochrome c release. The localization of cytochrome c was analyzed in noninduced (OFF) and induced (ON) HSP70 (clone 8-17) cells before (control) and after (HS) heat shock (43°C for 60 min followed by 6 h at 37°C). Cytochrome c has a punctate localization in control cells (A and E). Following a heat shock the mitochondrion-localized immunofluorescence pattern is lost and cytochrome c becomes evenly distributed throughout the cytoplasm (C). Cytochrome c remains localized to mitochondria in cells expressing hsp70 (G). Nuclei were stained with DAPI (B, D, F, and H).
FIG. 14
The chaperone function of hsp70 is required to block cytochrome c release. Cytochrome c release was analyzed in noninduced (OFF) and induced (ON) cells expressing hsp70 (clone 8-17) and hsp70ΔATPase by immunocytochemistry as described for Fig. 13. The cells with mitochondrion-localized or cytoplasmic cytochrome c were counted, and the percentages of cells with mitochondrion-localized cytochrome c are plotted. Punct, punctate.
Similar articles
- Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation.
Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Li CY, et al. J Biol Chem. 2000 Aug 18;275(33):25665-71. doi: 10.1074/jbc.M906383199. J Biol Chem. 2000. PMID: 10806214 - Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis.
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Mosser DD, et al. Mol Cell Biol. 1997 Sep;17(9):5317-27. doi: 10.1128/MCB.17.9.5317. Mol Cell Biol. 1997. PMID: 9271409 Free PMC article. - Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis.
Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Gabai VL, et al. Mol Cell Biol. 2002 May;22(10):3415-24. doi: 10.1128/MCB.22.10.3415-3424.2002. Mol Cell Biol. 2002. PMID: 11971973 Free PMC article. - [Heat shock protein 70 binding protein 1 induces enhanced apoptotic response against anticancer drugs in tumor cells].
Tanimura S, Kohno M. Tanimura S, et al. Nihon Rinsho. 2004 Jul;62(7):1291-6. Nihon Rinsho. 2004. PMID: 15283146 Review. Japanese. - Proteases for cell suicide: functions and regulation of caspases.
Chang HY, Yang X. Chang HY, et al. Microbiol Mol Biol Rev. 2000 Dec;64(4):821-46. doi: 10.1128/MMBR.64.4.821-846.2000. Microbiol Mol Biol Rev. 2000. PMID: 11104820 Free PMC article. Review.
Cited by
- Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways.
Beere HM. Beere HM. J Clin Invest. 2005 Oct;115(10):2633-9. doi: 10.1172/JCI26471. J Clin Invest. 2005. PMID: 16200196 Free PMC article. Review. - TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity.
Woo SK, Lee SD, Na KY, Park WK, Kwon HM. Woo SK, et al. Mol Cell Biol. 2002 Aug;22(16):5753-60. doi: 10.1128/MCB.22.16.5753-5760.2002. Mol Cell Biol. 2002. PMID: 12138186 Free PMC article. - More than one way to go.
Wyllie AH, Golstein P. Wyllie AH, et al. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):11-3. doi: 10.1073/pnas.98.1.11. Proc Natl Acad Sci U S A. 2001. PMID: 11136242 Free PMC article. No abstract available. - Identification and optimization of classifier genes from multi-class earthworm microarray dataset.
Li Y, Wang N, Perkins EJ, Zhang C, Gong P. Li Y, et al. PLoS One. 2010 Oct 28;5(10):e13715. doi: 10.1371/journal.pone.0013715. PLoS One. 2010. PMID: 21060837 Free PMC article.
References
- Beere H M, Wolf B B, Cain K, Mosser D D, Mahboubi A, Kuwana T, Taylor P, Morimoto R I, Cohen G, Green D R. Heat shock protein 70 (HSP70) inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. - PubMed
- Bornman L, Steinmann C M, Gericke G S, Polla B S. In vivo heat shock protects rat myocardial mitochondria. Biochem Biophys Res Commun. 1998;246:836–840. - PubMed
- Brar B K, Stephanou A, Wagstaff M J, Coffin R S, Marber M S, Engelmann G, Latchman D S. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–146. - PubMed
- Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. - PubMed
- Buzzard K A, Giaccia A J, Killender M, Anderson R L. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous