Isoforms of vascular endothelial growth factor act in a coordinate fashion To recruit and expand tumor vasculature - PubMed (original) (raw)
Isoforms of vascular endothelial growth factor act in a coordinate fashion To recruit and expand tumor vasculature
J Grunstein et al. Mol Cell Biol. 2000 Oct.
Abstract
Vascular endothelial growth factor (VEGF) is an essential regulator of vascularization. It is expressed as several splice variants; the major forms contain 120 amino acids, 164 amino acids, and 188 amino acids. We utilized transformed cells nullizygous for VEGF to specifically express each of these isoforms in isolation, in order to determine the role of each in tumorigenic neo-vascularization. We found that only the intermediate isoform, VEGF164, could fully rescue tumor growth; VEGF120 partially rescued tumor growth, and VEGF188 failed completely to rescue tumor expansion. Surprisingly, the vascular density of VEGF188 isoform-expressing tumors is significantly greater than that of wild-type VEGF cells and the other isoform-specific tumors. The failure of the hypervascular VEGF188-expressing tumors to grow may be due to inadequate perfusion of the massive number of microvessels in these tumors; three-dimensional imaging of the tumorigenic vasculature indicated little or no recruitment of the peripheral vasculature. This demonstrates that the VEGF isoforms perform unique functions which together enable tumorigenic vascularization.
Figures
FIG. 1
Expression levels in VEGF isoform-specific cell lines. (a) Northern blot analysis from retrovirally infected cell lines. Note the similar levels of isoform-specific RNA (5.0 to 5.2 kb). The cellular VEGF RNA (3.6 kb) is slightly smaller in the VEGF-null cell lines compared to that of wild type (WT) (3.7 kb) due to the absence of exon 2. The RNA filter was simultaneously probed with a fragment of GAPDH to control for gel loading differences. (b) Comparison of VEGF protein levels in conditioned medium from cell lines infected with isoform expressing adenoviruses. Cell lines were metabolically labeled and treated with heparin (100 μg/ml). (c) Comparison of cell growth rates of retrovirally infected, isoform-expressing cell lines and controls in tissue culture. (d) Northern blot analysis of neuropilin-1 (NP-1) in VEGF isoform-specific cell lines indicates similar expression levels of this receptor in each cell line used to generate tumors.
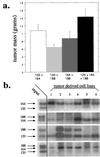
FIG. 2
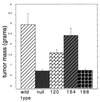
Tumorigenesis assay of VEGF isoform-specific cell lines. Shown is a comparison of the masses of fibrosarcomas generated from isoform-specific, stable cell lines and controls. A total of 107 cells in 100 μl of DMEM were injected subcutaneously, intracapularly into immunocompromised mice. Tumors were harvested and weighed 16 days postinjection (n = 9 animals per cell line). Error bars indicate 1 standard error.
FIG. 3
Immunostaining of vascular endothelium from VEGF isoform-specific fibrosarcomas. (a) Representative anti-CD31 immunohistology of tumor cryosections from wild-type transformed fibroblasts. (b) The same cells following cre-mediated excision of the VEGF gene. (c) VEGF120-expressing tumors. (d) VEGF164-expressing tumors. (e) VEGF-188 expressing tumors. Magnification, ×100.
FIG. 4
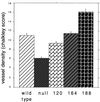
Microvessel density quantification by Chalkley analysis. For each tumor type, 10 representative fields from each of 3 individual tumors were scored for the maximum overlap of stained vessels with the random spot array on the Chalkley graticule. Error bars indicate 1 standard error.
FIG. 5
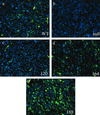
VEGF immunostaining from paraffin-embedded tumor sections demonstrates relative local concentration of VEGF isoforms. (a) Wild-type control; (b) VEGF-deficient control; (c) VEGF120; (d) VEGF164; (e) VEGF188. Fluorescein isothiocyanate-conjugated secondary Ab recognizes anti-VEGF Ab-3 (JH121). Nuclear counterstain is Hoechst 33342 (blue). Magnification, ×400. Note the high degree of cell-associated VEGF staining in VEGF188-expressing tumors compared to the strictly vessel-associated staining found in VEGF120-expressing tumors.
FIG. 6
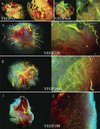
Macroscopic view of tumor vasculature and surrounding stroma. Perfusion of tumor-bearing mice with liquid latex (Microfil) compound results in a three-dimensional cast of major vessels and microvessels. (a and b) Wild-type control tumor at magnifications of ×7 and ×20. (c and d) VEGF-null tumor at similar magnifications. (e and f) VEGF120. Note the extreme degree of host vasculature localized to the periphery of the tumor (arrows) and poor infiltration, coincident with the low local concentration of VEGF in the tumor. (g and h) VEGF164. Note the high degree of systemic vasculature directed to the tumor as well as significant microvasculature. (i and j) VEGF188. Note the presence of fewer systemic vessels directed to the tumor yet many microvessels within the tumor itself.
FIG. 7
Tumor microenvironment selects for a gradient of VEGF expression. (a) Tumor masses resulting from subcutaneous injection of cell lines composed of every combination of isoform-specific cell lines. Note that there is a slight growth advantage to tumors which express all isoforms. (b) Southern blot analysis of isoform-specific VEGF levels in cell lines harvested from representative tumors in panel a. Input lanes represent the equal contribution from each cell line prior to tumor formation. Note the competitive advantage of VEGF164-expressing cells and the decrease in selective pressure in tumors expressing only VEGF120 and VEGF188, indicating that the tumor requires both soluble and cell-associated VEGF.
FIG. 8
Proposed gradient model of tumorigenic VEGF signaling. VEGF120 produces a diffuse signal (light blue) which recruits peripheral vessels but does little to vascularize the tumor itself. VEGF164 can both recruit vessels with a partially diffusible signal and vascularize the tumor with a partially cell-associated signal. VEGF188 fails to adequately recruit the host vasculature, but vascular endothelium which is captured forms a hypervascular capillary network due to the high local concentration of VEGF.
Similar articles
- The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo.
Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, Harris AL, Ziche M, Bicknell R. Zhang HT, et al. Br J Cancer. 2000 Jul;83(1):63-8. doi: 10.1054/bjoc.2000.1279. Br J Cancer. 2000. PMID: 10883669 Free PMC article. - Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188.
Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G. Maes C, et al. Mech Dev. 2002 Feb;111(1-2):61-73. doi: 10.1016/s0925-4773(01)00601-3. Mech Dev. 2002. PMID: 11804779 - Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.
Stalmans I. Stalmans I. Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review. - Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors.
Tozer GM, Akerman S, Cross NA, Barber PR, Björndahl MA, Greco O, Harris S, Hill SA, Honess DJ, Ireson CR, Pettyjohn KL, Prise VE, Reyes-Aldasoro CC, Ruhrberg C, Shima DT, Kanthou C. Tozer GM, et al. Cancer Res. 2008 Apr 1;68(7):2301-11. doi: 10.1158/0008-5472.CAN-07-2011. Cancer Res. 2008. PMID: 18381437 - VEGF in brain tumors.
Machein MR, Plate KH. Machein MR, et al. J Neurooncol. 2000 Oct-Nov;50(1-2):109-20. doi: 10.1023/a:1006416003964. J Neurooncol. 2000. PMID: 11245271 Review.
Cited by
- Neuroprotection using gene therapy to induce vascular endothelial growth factor-A expression.
Sakowski SA, Heavener SB, Lunn JS, Fung K, Oh SS, Spratt SK, Hogikyan ND, Feldman EL. Sakowski SA, et al. Gene Ther. 2009 Nov;16(11):1292-9. doi: 10.1038/gt.2009.111. Epub 2009 Sep 3. Gene Ther. 2009. PMID: 19727131 Free PMC article. - Vascular endothelial growth factor reduces mural cell coverage of endothelial cells and induces sprouting rather than luminal division in an HT1080 tumour angiogenesis model.
Fujimoto A, Onodera H, Mori A, Isobe N, Yasuda S, Oe H, Yonenaga Y, Tachibana T, Imamura M. Fujimoto A, et al. Int J Exp Pathol. 2004 Dec;85(6):355-64. doi: 10.1111/j.0959-9673.2004.00404.x. Int J Exp Pathol. 2004. PMID: 15566432 Free PMC article. - Cancer-triggered systemic disease and therapeutic targets.
Cao Y. Cao Y. Holist Integr Oncol. 2024;3(1):11. doi: 10.1007/s44178-024-00077-w. Epub 2024 Mar 11. Holist Integr Oncol. 2024. PMID: 38482486 Free PMC article. - Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors.
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Lee S, et al. J Cell Biol. 2005 May 23;169(4):681-91. doi: 10.1083/jcb.200409115. J Cell Biol. 2005. PMID: 15911882 Free PMC article. - Therapeutic angiogenesis for cardiovascular disease.
Ng YS, D'Amore PA. Ng YS, et al. Curr Control Trials Cardiovasc Med. 2001;2(6):278-285. doi: 10.1186/cvm-2-6-278. Curr Control Trials Cardiovasc Med. 2001. PMID: 11806814 Free PMC article.
References
- Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. - PMC - PubMed
- Bacic M, Edwards N A, Merrill M J. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissues. Growth Factors. 1995;12:11–15. - PubMed
- Banga J D. Therapeutic angiogenesis through intramuscular injection of the gene for vascular endothelial growth factor (VEGF) Ned Tijdschr Geneeskd. 2000;144:113–116. . (In Dutch.) - PubMed
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
- Carmeliet P, Ng Y S, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar V V, Stalmans I, Mattot V, Perriard J C, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D'Amore P A, Shima D T. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous