Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription - PubMed (original) (raw)
. 2000 Sep 15;19(18):4976-85.
doi: 10.1093/emboj/19.18.4976.
C Mirtsos, S Suzuki, K Graham, J Huang, M Ng, A Itié, A Wakeham, A Shahinian, W J Henzel, A J Elia, W Shillinglaw, T W Mak, Z Cao, W C Yeh
Affiliations
- PMID: 10990461
- PMCID: PMC314216
- DOI: 10.1093/emboj/19.18.4976
Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription
M Bonnard et al. EMBO J. 2000.
Abstract
Induction of NF-kappaB-dependent transcription requires phosphorylation and subsequent degradation of I-kappaB, an inhibitor of NF-kappaB, followed by nuclear translocation and DNA binding of NF-kappaB. Tumor necrosis factor receptor-associated factor 2 (TRAF2) plays a role in NF-kappaB activation in response to cytokines such as tumor necrosis factor alpha (TNFalpha). In this study, we purified and characterized a novel kinase (T2K, also known as TBK1 or NAK), which associates with TRAF2 and exhibits kinase activity towards I-kappaBalpha in vitro. The physiological function of T2K was investigated using T2K-deficient mice. Heterozygotes appear normal, but t2k(-/-) animals die at approximately E14.5 of massive liver degeneration and apoptosis. Never theless, hematopoietic progenitors from T2K-deficient fetal liver support normal lymphocyte development. Furthermore, t2k(-/-) embryonic fibroblasts and thymocytes do not display increased sensitivity to TNFalpha-induced apoptosis. In response to either TNFalpha or IL-1 induction, t2k(-/-) embryonic fibroblasts exhibit normal degradation of I-kappaB and kappaB-binding activity. However, NF-kappaB-directed transcription is dramatically reduced. These results demonstrate that, like I-kappaB kinase beta and the RelA subunit of NF-kappaB, T2K is critical in protecting embryonic liver from apoptosis. However, T2K has a unique role in the activation of NF-kappaB-directed transcription, apparently independent of I-kappaB degradation and NF-kappaB DNA binding.
Figures
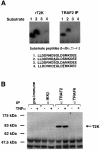
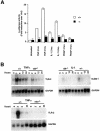
Fig. 1. Identification of a TRAF2-associated kinase (T2K). (A) TRAF2 complexes or recombinant T2K were used in in vitro kinase assays to assess the phosphorylation of peptide substrates encompassing amino acids 25–41 of either wild-type or mutated I-κBα (mutated amino acids are underlined). (B) Co-immunoprecipitation of endogenous T2K. Extracts of 293 cells either left untreated or stimulated with 100 ng/ml TNFα for 10 min were incubated with pre-immune rabbit serum or rabbit anti-serum specific for either IKKβ, TRAF2 or TRAF6 monoclonal antibody followed by western blotting using antiserum specific for recombinant T2K.
Fig. 2. Targeted disruption of the murine t2k gene. (A) Top, a portion of the endogenous t2k locus containing three exons (shown as solid boxes). Middle, the targeting construct. Bottom, the mutant locus resulting from homologous recombination. The _Sac_I (S) restriction site and the flanking probe used to detect homologous recombination are indicated. (B) Genomic Southern blot of _Sac_I-digested DNA from E13.5 embryos of the genotypes indicated, showing the 6.6 kb wild-type band and the 14.6 kb mutant band detected by the flanking probe in (A). (C) Genotyping by PCR of DNA from E13.5 embryos using the primers described in Materials and methods. (D) Northern blot analysis of T2K RNA expression in E13.5 EFs of the genotypes indicated. The cells were either left untreated or stimulated with 10 ng/ml TNFα for the time points indicated.
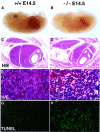
Fig. 3. Massive liver apoptosis in the absence of T2K. (A and B) Low power view of E14.5 wild-type (A) and _t2k_–/– (B) embryos. (C–F) Histological analysis of E14.5 liver sections. Views of H&E staining of wild-type (C and E) and mutant (D and F) livers at either low (C and D) or high (E and F) magnification are shown. (G and H) Enhanced apoptosis in livers of E14.5 T2K-deficient embryos. TUNEL assays were performed on sections of wild-type (G) and mutant (H) E14.5 embryos. A high power view of the liver is shown in both cases.
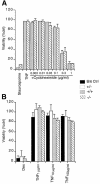
Fig. 4. Normal sensitivity of thymocytes and EFs to TNFα-induced cell death in the absence of T2K. (A) TNFα-induced apoptosis of EFs. Wild-type and T2K-deficient EFs were either left untreated or stimulated with staurosporine (2 µM) or TNFα (10 ng/ml) in combination with the indicated concentrations of cycloheximide for 18 h. Cells were harvested and stained for viability as described in Materials and methods. Values shown are the percentage of viable cells after treatment relative to untreated controls. Individual treatments were measured in triplicate. (B) TNFα-induced apoptosis of CD4+CD8+ (DP) thymocytes. Thymocytes from the reconstituted mice described in the text were cultured at 37°C for 20 h in the presence of either 1 µM dexamethasone or the indicated concentrations of TNFα. Cell viability was determined by 7-amino actinomycin D (7-AAD) staining. Results are expressed as the percentage of total viable DP cells remaining after treatment relative to the untreated control of the same genotype. Individual treatments were measured in quadruplicate.
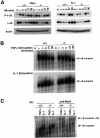
Fig. 5. Normal I-κBα phosphorylation, degradation and NF-κB DNA binding activity in _t2k_–/– EFs. (A) I-κBα phosphorylation/degradation. Wild-type and T2K-deficient EFs were treated with either TNFα (10 ng/ml) or IL-1β (10 ng/ml) for the times indicated. Cytoplasmic extracts were analyzed by western blotting using monoclonal antibodies specific for either phospho-I-κBα (top), I-κBα (middle) or the loading control actin (bottom). (B) κB binding activity. Wild-type and _t2k_–/– EFs were incubated with either mouse recombinant TNFα (10 ng/ml) (top) or IL-1β (10 ng/ml) (bottom) for the times indicated. NF-κB activation in 10 µg of nuclear extract was determined by EMSA as described in Materials and methods. For both (A) and (B), one result representative of three independent experiments is shown. (C) RelA binding activity. Wild-type and _t2k_–/– EFs were incubated with 10 ng/ml of either mouse recombinant TNFα or IL-1β for 27 min. RelA binding activity was assessed by incubation of 10 µg of nuclear extract with RelA-specific antibody followed by electrophoretic mobility supershift assay, as described in Materials and methods.
Fig. 6. Impaired NF-κB-dependent gene expression in _t2k_–/– EFs. (A) Impaired transactivation of NF-κB-dependent reporter activity in _t2k_–/– EFs. Wild-type and _t2k_–/– EFs were transfected with the pELAM-luc reporter and CMV-lacZ vectors. After stimulation with 10 ng/ml TNFα or IL-1, or 100 ng/ml PDGF for the times indicated, lysates were assayed for luciferase activity as described in Materials and methods. Individual treatments were measured in triplicate, and results representative of four independent experiments are shown. (B) Impaired induction of the endogenous NF-κB target genes ICAM-1 and TLR-2. Wild-type and _t2k_–/– EFs were stimulated with 10 ng/ml TNFα or IL-1 for the times indicated followed by northern blot analysis of 20 µg of total RNA using a 32P-labeled random priming probe corresponding to the full-length ICAM-1 or TLR-2 cDNA. GAPDH expression was analyzed to control for RNA loading. In the bottom panel, wild-type and _relA_–/– EFs were stimulated with 10 ng/ml for the times indicated followed by northern blot analysis of 2 µg of mRNA, as above.
Similar articles
- Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review. - Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice.
Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Rosenfeld ME, et al. Am J Pathol. 2000 Mar;156(3):997-1007. doi: 10.1016/S0002-9440(10)64967-X. Am J Pathol. 2000. PMID: 10702415 Free PMC article. - Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway.
Leitges M, Sanz L, Martin P, Duran A, Braun U, García JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Leitges M, et al. Mol Cell. 2001 Oct;8(4):771-80. doi: 10.1016/s1097-2765(01)00361-6. Mol Cell. 2001. PMID: 11684013 - NF-kappaB activation and HIV-1 induced apoptosis.
DeLuca C, Kwon H, Lin R, Wainberg M, Hiscott J. DeLuca C, et al. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):235-53. doi: 10.1016/s1359-6101(99)00015-5. Cytokine Growth Factor Rev. 1999. PMID: 10647779 Review.
Cited by
- Role of TBK1 Inhibition in Targeted Therapy of Cancer.
Yang X, Liu Z. Yang X, et al. Mini Rev Med Chem. 2024;24(10):1031-1045. doi: 10.2174/0113895575271977231115062803. Mini Rev Med Chem. 2024. PMID: 38314681 Review. - IκB kinase ε (IKKε): a therapeutic target in inflammation and cancer.
Verhelst K, Verstrepen L, Carpentier I, Beyaert R. Verhelst K, et al. Biochem Pharmacol. 2013 Apr 1;85(7):873-80. doi: 10.1016/j.bcp.2013.01.007. Epub 2013 Jan 17. Biochem Pharmacol. 2013. PMID: 23333767 Free PMC article. Review. - The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis.
Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, Young HA, Ching LM, Vogel SN. Roberts ZJ, et al. J Exp Med. 2007 Jul 9;204(7):1559-69. doi: 10.1084/jem.20061845. Epub 2007 Jun 11. J Exp Med. 2007. PMID: 17562815 Free PMC article. - Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity.
tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Yamamoto M, Akira S, Yeh WC, Lin R, Hiscott J. tenOever BR, et al. J Virol. 2004 Oct;78(19):10636-49. doi: 10.1128/JVI.78.19.10636-10649.2004. J Virol. 2004. PMID: 15367631 Free PMC article. - Signaling for survival and apoptosis in the immune system.
Mak TW, Yeh WC. Mak TW, et al. Arthritis Res. 2002;4 Suppl 3(Suppl 3):S243-52. doi: 10.1186/ar569. Epub 2002 May 9. Arthritis Res. 2002. PMID: 12110144 Free PMC article. Review.
References
- Amakawa R. et al. (1996) Impaired negative selection of T cells in Hodgkin’s disease antigen CD30-deficient mice. Cell, 84, 551–562. - PubMed
- Anrather J., Csizmadia,V., Soares,M.P. and Winkler,H. (1999) Regulation of NF-κB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Cζ in primary endothelial cells. J. Biol. Chem., 274, 13594–13603. - PubMed
- Arch R.H., Gedrich,R.W. and Thompson,C.B. (1998) Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. - PubMed
- Beg A.A. and Baltimore,D. (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science, 274, 782–784. - PubMed
- Beg A.A., Sha,W.C., Bronson,R.T., Ghosh,S. and Baltimore,D. (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kB. Nature, 376, 167–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous