Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells - PubMed (original) (raw)
Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells
H Hochrein et al. J Exp Med. 2000.
Abstract
Interleukin (IL)-12 may be secreted as a bioactive T helper type 1 (Th1) cell-inducing heterodimer, as a monomer, or as an antagonistic homodimer. We analyzed the IL-12 produced by mouse splenic dendritic cells (DCs), human thymic DCs, and cultured human monocyte-derived DCs. IL-12 production required both a microbial or T cell-derived stimulus and an appropriate cytokine milieu. The different IL-12 forms were differentially regulated by the cytokines present rather than the stimulus used. IL-4 alone or together with granulocyte/macrophage colony-stimulating factor or interferon gamma effectively enhanced the production of the bioactive heterodimer and selectively reduced the antagonistic homodimer of IL-12. Therefore, IL-4, the major Th2-driving cytokine, provides a negative feedback causing DCs to produce the major Th1-inducing cytokine, bioactive IL-12.
Figures
Figure 1
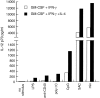
IL-4 enhances IL-12 p70 production by mouse splenic DCs. Mouse splenic DCs were cultured with an IL-12 stimulus (LPS, anti-CD40 mAb, poly I:C, CpG, or SAC [10 μg/ml], or a mix of all stimuli together) in the presence of GM-CSF and IFN-γ with or without IL-4, for 18 h. The supernatants were assayed for IL-12 p70 content by ELISA. The result is typical of more than five experiments of this type.
Figure 2
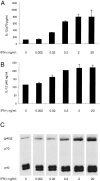
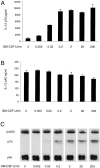
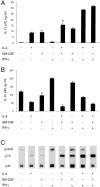
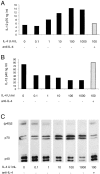
Titration of the effect of IFN-γ on the production of IL-12 by mouse splenic DCs. Mouse splenic DCs were cultured with SAC (20 μg/ml) in the presence of titrated concentrations of IFN-γ, for 23 h. The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The error bars represent the variation within one typical experiment. The experiment has been repeated three times for the ELISA and two times for Western blots, with similar results.
Figure 3
Titration of the effect of GM-CSF on the production of IL-12 by mouse splenic DCs. Mouse splenic DCs were cultured with SAC (20 μg/ml) in the presence of IFN-γ and titrated concentrations of GM-CSF, for 23 h. The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The result is typical of three experiments with ELISA readouts and Western blotting.
Figure 5
The effects of combinations of IL-4, GM-CSF, and IFN-γ on the IL-12 production by mouse splenic DCs. Mouse splenic DCs were cultured with a mix of stimuli as used in Fig. 1 in the presence or the absence of IL-4, IFN-γ, or GM-CSF, for 23 h. The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The result is typical of more than five similar experiments with ELISA readouts and Western blotting.
Figure 4
Titration of the effect of IL-4 on the production of IL-12 by mouse splenic DCs. Mouse splenic DCs were cultured with a mix of stimuli as used in Fig. 1 (but with SAC used at only 5 μg/ml), in the presence of IFN-γ and GM-CSF and titrated concentrations of IL-4, for 18 h. A neutralizing mAb to IL-4 (10 μg/ml) was included for one set of conditions as indicated (gray bar). The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The result is typical of three similar experiments with ELISA readouts and Western blotting.
Figure 6
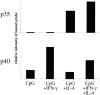
The effects of IL-4 on p70 expression are mediated by the regulation of p35 mRNA. Mouse splenic DCs were cultured with CpG alone or with the addition of IFN-γ, IL-4, or both, for 4 h. RNA was extracted from the cells and subjected to Northern transfer analysis. The relative amounts of radioactive p35-specific or p40-specific probes bound to the mRNA samples as quantitated using a PhosphorImager® (Molecular Probes) are shown. A repeat experiment confirmed these findings.
Similar articles
- Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy.
Feili-Hariri M, Falkner DH, Morel PA. Feili-Hariri M, et al. J Leukoc Biol. 2005 Sep;78(3):656-64. doi: 10.1189/jlb.1104631. Epub 2005 Jun 16. J Leukoc Biol. 2005. PMID: 15961574 - IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.
Strengell M, Lehtonen A, Matikainen S, Julkunen I. Strengell M, et al. J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679 - Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha.
Della Bella S, Nicola S, Riva A, Biasin M, Clerici M, Villa ML. Della Bella S, et al. J Leukoc Biol. 2004 Jan;75(1):106-16. doi: 10.1189/jlb.0403154. Epub 2003 Oct 2. J Leukoc Biol. 2004. PMID: 14525963 - Estrogen inhibition of EAE involves effects on dendritic cell function.
Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark AA, Offner H. Liu HY, et al. J Neurosci Res. 2002 Oct 15;70(2):238-48. doi: 10.1002/jnr.10409. J Neurosci Res. 2002. PMID: 12271473 - B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation.
Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. Moulin V, et al. J Exp Med. 2000 Aug 21;192(4):475-82. doi: 10.1084/jem.192.4.475. J Exp Med. 2000. PMID: 10952717 Free PMC article.
Cited by
- Self-reactivity as the necessary cost of maintaining a diverse memory T-cell repertoire.
Singh NJ. Singh NJ. Pathog Dis. 2016 Oct;74(7):ftw092. doi: 10.1093/femspd/ftw092. Epub 2016 Sep 11. Pathog Dis. 2016. PMID: 27620200 Free PMC article. Review. - Specific microtubule-depolymerizing agents augment efficacy of dendritic cell-based cancer vaccines.
Wen CC, Chen HM, Chen SS, Huang LT, Chang WT, Wei WC, Chou LC, Arulselvan P, Wu JB, Kuo SC, Yang NS. Wen CC, et al. J Biomed Sci. 2011 Jun 20;18(1):44. doi: 10.1186/1423-0127-18-44. J Biomed Sci. 2011. PMID: 21689407 Free PMC article. - Immunity to Pathogens Taught by Specialized Human Dendritic Cell Subsets.
Geginat J, Nizzoli G, Paroni M, Maglie S, Larghi P, Pascolo S, Abrignani S. Geginat J, et al. Front Immunol. 2015 Oct 13;6:527. doi: 10.3389/fimmu.2015.00527. eCollection 2015. Front Immunol. 2015. PMID: 26528289 Free PMC article. Review. - Leucocyte-associated immunoglobulin-like receptor-1 is an inhibitory regulator of contact hypersensitivity.
Omiya R, Tsushima F, Narazaki H, Sakoda Y, Kuramasu A, Kim Y, Xu H, Tamura H, Zhu G, Chen L, Tamada K. Omiya R, et al. Immunology. 2009 Dec;128(4):543-55. doi: 10.1111/j.1365-2567.2009.03140.x. Immunology. 2009. PMID: 19930044 Free PMC article. - Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas.
Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, Hamilton RL, Torres-Trejo A, Kalinski P, Cai Q, Mabold JL, Edington HD, Butterfield LH, Whiteside TL, Potter DM, Schold SC Jr, Pollack IF. Okada H, et al. J Transl Med. 2007 Dec 19;5:67. doi: 10.1186/1479-5876-5-67. J Transl Med. 2007. PMID: 18093335 Free PMC article. Clinical Trial.
References
- Kobayashi M., Fitz L., Ryan M., Hewick R.M., Clark S.C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170:827–845. - PMC - PubMed
- Wolf S.F., Temple P.A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R.M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 1991;146:3074–3081. - PubMed
- Gubler U., Chua A.O., Schoenhaut D.S., Dwyer C.M., McComas W., Motyka R., Nabavi N., Wolitzky A.G., Quinn P.M., Familletti P.C. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. USA. 1991;88:4143–4147. - PMC - PubMed
- Trinchieri G. Interleukin-12a cytokine at the interface of inflammation and immunity. Adv. Immunol. 1998;70:83–243. - PubMed
- Gately M.K., Renzetti L.M., Magram J., Stern A.S., Adorini L., Gubler U., Presky D.H. The interleukin-12/interleukin-12-receptor systemrole in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources