Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex - PubMed (original) (raw)
Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex
H N Higgs et al. J Cell Biol. 2000.
Abstract
We purified native WASp (Wiskott-Aldrich Syndrome protein) from bovine thymus and studied its ability to stimulate actin nucleation by Arp2/3 complex. WASp alone is inactive in the presence or absence of 0.5 microM GTP-Cdc42. Phosphatidylinositol 4,5 bisphosphate (PIP(2)) micelles allowed WASp to activate actin nucleation by Arp2/3 complex, and this was further enhanced twofold by GTP-Cdc42. Filaments nucleated by Arp2/3 complex and WASp in the presence of PIP(2) and Cdc42 concentrated around lipid micelles and vesicles, providing that Cdc42 was GTP-bound and prenylated. Thus, the high concentration of WASp in neutrophils (9 microM) is dependent on interactions with both acidic lipids and GTP-Cdc42 to activate actin nucleation by Arp2/3 complex. The results also suggest that membrane binding increases the local concentrations of Cdc42 and WASp, favoring their interaction.
Figures
Figure 1
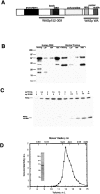
Extraction and purification of WASp from leukocytes. (A) Schematic diagram of WASp with the domains and constructs used in this study. (B) Extraction of WASp. Homogenates (Homog.) from human peripheral neutrophils and bovine thymus were fractionated into a 4,000_-g_ pellet (LSP), 100,000_-g_ pellet (HSP), and 100,000_-g_ supernatant (HSS). 10 μg of the total homogenate and equal volumes of the centrifuged fractions were Western blotted with AB1. (C) Determination of WASp concentration in human neutrophils. SDS-PAGE of homogenates at various dilutions in the absence or presence of varying concentrations of WASp152-309. Western blots against AB1 were analyzed by densitometry. (D) Gel filtration of purified WASp on Superdex200 in 10 mM imidazole, pH 7.0, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, and 1 mM DTT. The concentrations of WASp in the eluted fractions were measured by quantitative Western blotting. (inset) Coomassie blue–stained SDS-PAGE of 0.2 μg purified WASp before gel filtration.
Figure 2
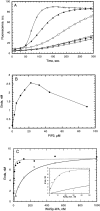
Activation of Arp2/3 complex by native WASp and WASp-WA. (A) Effect of Cdc42 and PIP2 on WASp activation of Arp2/3 complex. Monomeric MgATP actin (4 μM, 5% pyrene-labeled) was polymerized in the presence of 10 nM of Arp2/3 complex and no addition (open circles), 4 nM WASp (filled circles), 4 nM WASp, and 500 nM prenylated GTPγS-Cdc42 (open diamonds), 4 nM WASp, and 20 μM PIP2 micelles (open squares), 4 nM WASp, 20 μM PIP2 micelles and 500 nM prenylated GTPγS-Cdc42 (filled triangles), and 500 nM WASp WA (crosses). (B) Dependence of nucleation by Arp2/3 complex and native WASp on PIP2 concentration. Conditions were as described in A, with 4 nM WASp and a varying concentration of PIP2 in the absence of Cdc42. The concentration of filament ends was calculated from the slopes of polymerization curves at 80% polymerization. (C) Dependence of nucleation by Arp2/3 complex on the concentrations of dimeric GST-WA (closed circles) or monomeric WASp-WA (open circles). Conditions are as in A, except no PIP2 or Cdc42 was added.
Figure 3
Fluorescence micrographs of the products of actin polymerization reactions. Polymerization reactions contained 4 μM monomeric MgATP actin, with or without 100 nM of Arp2/3 complex, 4 nM WASp, 0.5 μM prenylated GTPγS-Cdc42, 22 μM PIP2 micelles, or 100 μM of phospholipid vesicles. After polymerization for 20 min at 23°C in KMEI containing 4 μM rhodamine-phalloidin, samples were diluted 625-fold (A–F) or 25-fold (G and H) into motility buffer, mounted on nitrocellulose-coated coverslips, and viewed with filters for rhodamine. (A) Arp2/3 complex, prenylated GTPγS-Cdc42, PIP2 micelles, (B) WASp, prenylated GTPγS-Cdc42, PIP2 micelles, (C) Arp2/3 complex, WASp, (D) Arp2/3 complex, WASp, PIP2 micelles, (E) Arp2/3 complex, WASp, prenylated GTPγS-Cdc42, (F) Arp2/3 complex, WASp, prenylated GTPγS-Cdc42, PIP2 micelles, (G) Arp2/3 complex, WASp, prenylated GTPγS-Cdc42, multilamellar vesicles containing 50% cholesterol/14.5% PC/14.5% PE/20% PS/1% CF-PE, or (H) Arp2/3 complex, WASp, prenylated GTPγS-Cdc42, multilamellar vesicles containing 50% cholesterol/19.5% PC/19.5% PE/10% PIP2/1% CF-PE. Inset in G shows corresponding fluorescein image of G, showing the vesicle at the interior of the actin halo (left) and a vesicle not surrounded by actin.
Figure 5
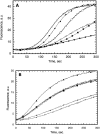
WASp152-309 interacts with GTPγS-Cdc42 but not with PIP2. (A) Effect of GTPγS-Cdc42 and PIP2 on nucleation inhibition by WASp152-309. Nucleation assays were performed as described in Fig. 2 A with 10 nM of Arp2/3 complex alone (filled circles); Arp2/3 complex, 250 nM WASp WA (open circles); Arp2/3 complex, WASp WA, 3 μM WASp152-309 (filled squares); Arp2/3 complex, WASp WA, WASp152-309, 1.6 μM prenylated GTPγS-Cdc42 (open squares); Arp2/3 complex, WASp WA, WASp152-309, 3.2 μM prenylated GTPγS-Cdc42 (filled diamonds); and Arp2/3 complex, WASp WA, WASp152-309, 30 μM PIP2 (open diamonds). (B) Effect of WASp152-309 on full-length native WASp activation by PIP2 and GTPγS-Cdc42. Nucleation assays were performed as described in Fig. 2 A with 10 nM of Arp2/3 complex alone (open circles); Arp2/3 complex, 2 nM WASp (filled circles); Arp2/3 complex, WASp, 20 μM PIP2 (open squares); Arp2/3 complex, WASp, PIP2, 25 μM WASp152-309 (filled squares); Arp2/3 complex, WASp + PIP2, 0.5 μM prenylated GTPγS-Cdc42 (filled triangles); and Arp2/3 complex, WASp, PIP2, prenylated GTPγS-Cdc42, 25 μM WASp152-309 (open triangles).
Figure 4
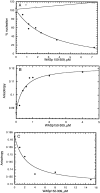
Regulation of WASp-WA by a fragment of WASp including the GBD. (A) WASp 152-309 inhibits Arp2/3 complex activation by WASp WA. Nucleation reactions included 4 μM of monomeric MgATP actin (5% pyrene-labeled), 10 nM of Arp2/3 complex, 250 nM WASp WA or Scar1 WA, and varying concentrations of WASp 152-309. The concentration of filament ends created by Arp2/3 complex was calculated from the slope of the polymerization curve at 80% polymerization. Percent nucleation is the concentration of ends created relative to that created by Arp2/3 complex and WASp WA or Scar1 WA in the absence of WASp 152-309, after subtraction of the concentration of filaments created in the absence of WA. (B) Binding of WASp 152-309 to WASp WA was measured by fluorescence anisotropy of rhodamine-labeled WASp WA (0.1 μM). The calculated dissociation equilibrium constant is 0.42 μM. (C) Fluorescence anisotropy assay for competition between Arp2/3 complex and WASp152-309 for binding WASp WA. Rhodamine-labeled WASp WA (0.1 μM) has an anisotropy of 0.162 when bound to 1 μM of Arp2/3 complex. The smaller WASp152-309 competes off Arp2/3 complex, giving an anisotropy of 0.132.
Comment in
- How WASP regulates actin polymerization.
Zigmond SH. Zigmond SH. J Cell Biol. 2000 Sep 18;150(6):F117-20. doi: 10.1083/jcb.150.6.f117. J Cell Biol. 2000. PMID: 10995455 Free PMC article. Review. No abstract available.
Similar articles
- Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate.
Rohatgi R, Ho HY, Kirschner MW. Rohatgi R, et al. J Cell Biol. 2000 Sep 18;150(6):1299-310. doi: 10.1083/jcb.150.6.1299. J Cell Biol. 2000. PMID: 10995436 Free PMC article. - A novel neural Wiskott-Aldrich syndrome protein (N-WASP) binding protein, WISH, induces Arp2/3 complex activation independent of Cdc42.
Fukuoka M, Suetsugu S, Miki H, Fukami K, Endo T, Takenawa T. Fukuoka M, et al. J Cell Biol. 2001 Feb 5;152(3):471-82. doi: 10.1083/jcb.152.3.471. J Cell Biol. 2001. PMID: 11157975 Free PMC article. - Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex.
Ho HY, Rohatgi R, Lebensohn AM, Le Ma, Li J, Gygi SP, Kirschner MW. Ho HY, et al. Cell. 2004 Jul 23;118(2):203-16. doi: 10.1016/j.cell.2004.06.027. Cell. 2004. PMID: 15260990 - Signalling to actin: the Cdc42-N-WASP-Arp2/3 connection.
Carlier MF, Ducruix A, Pantaloni D. Carlier MF, et al. Chem Biol. 1999 Sep;6(9):R235-40. doi: 10.1016/s1074-5521(99)80107-0. Chem Biol. 1999. PMID: 10467124 Review. - [Reorganization of the actin cytoskeleton by WASP family proteins].
Miki H. Miki H. Seikagaku. 2002 Sep;74(9):1149-61. Seikagaku. 2002. PMID: 12402455 Review. Japanese. No abstract available.
Cited by
- Immune pathology associated with altered actin cytoskeleton regulation.
Wickramarachchi DC, Theofilopoulos AN, Kono DH. Wickramarachchi DC, et al. Autoimmunity. 2010 Feb;43(1):64-75. doi: 10.3109/08916930903374634. Autoimmunity. 2010. PMID: 20001423 Free PMC article. Review. - Phosphoinositides and Rho proteins spatially regulate actin polymerization to initiate and maintain directed movement in a one-dimensional model of a motile cell.
Dawes AT, Edelstein-Keshet L. Dawes AT, et al. Biophys J. 2007 Feb 1;92(3):744-68. doi: 10.1529/biophysj.106.090514. Epub 2006 Nov 10. Biophys J. 2007. PMID: 17098793 Free PMC article. - Proteome analysis of cultivated vascular smooth muscle cells from a CADASIL patient.
Ihalainen S, Soliymani R, Iivanainen E, Mykkänen K, Sainio A, Pöyhönen M, Elenius K, Järveläinen H, Viitanen M, Kalimo H, Baumann M. Ihalainen S, et al. Mol Med. 2007 May-Jun;13(5-6):305-14. doi: 10.2119/2006–00069.Ihalainen. Mol Med. 2007. PMID: 17622327 Free PMC article. - Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein)-family proteins and the Arp2/3 complex.
Millard TH, Sharp SJ, Machesky LM. Millard TH, et al. Biochem J. 2004 May 15;380(Pt 1):1-17. doi: 10.1042/BJ20040176. Biochem J. 2004. PMID: 15040784 Free PMC article. Review. - c-mip impairs podocyte proximal signaling and induces heavy proteinuria.
Zhang SY, Kamal M, Dahan K, Pawlak A, Ory V, Desvaux D, Audard V, Candelier M, BenMohamed F, Matignon M, Christov C, Decrouy X, Bernard V, Mangiapan G, Lang P, Guellaën G, Ronco P, Sahali D. Zhang SY, et al. Sci Signal. 2010 May 18;3(122):ra39. doi: 10.1126/scisignal.2000678. Sci Signal. 2010. PMID: 20484117 Free PMC article.
References
- Abdul-Manan N., Aghazadeh B., Liu G.A., Majumdar A., Ouerfelli O., Siminovitch K.A., Rosen M.K. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich Syndrome’ protein. Nature. 1999;399:379–383. - PubMed
- Blanchoin L., Pollard T.D. Interaction of actin monomers with Acanthamoeba actophorin (ADF/cofilin) and profilin. J. Biol. Chem. 1998;273:25106–25111. - PubMed
- Blanchoin L., Amann K.J., Higgs H.N., Marchand J.B., Kaiser D.A., Pollard T.D. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASp/Scar proteins. Nature. 2000;404:1007–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000833/RR/NCRR NIH HHS/United States
- CA14195/CA/NCI NIH HHS/United States
- RR00833/RR/NCRR NIH HHS/United States
- GM26338/GM/NIGMS NIH HHS/United States
- R01 GM026338/GM/NIGMS NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous