Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts - PubMed (original) (raw)
Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts
A Wodarz et al. J Cell Biol. 2000.
Erratum in
- J Cell Biol. 2004 May 24;165(4):following 589
Abstract
The establishment and maintenance of polarity is of fundamental importance for the function of epithelial and neuronal cells. In Drosophila, the multi-PDZ domain protein Bazooka (Baz) is required for establishment of apico-basal polarity in epithelia and in neuroblasts, the stem cells of the central nervous system. In the latter, Baz anchors Inscuteable in the apical cytocortex, which is essential for asymmetric localization of cell fate determinants and for proper orientation of the mitotic spindle. Here we show that Baz directly binds to the Drosophila atypical isoform of protein kinase C and that both proteins are mutually dependent on each other for correct apical localization. Loss-of-function mutants of the Drosophila atypical isoform of PKC show loss of apico-basal polarity, multilayering of epithelia, mislocalization of Inscuteable and abnormal spindle orientation in neuroblasts. Together, these data provide strong evidence for the existence of an evolutionary conserved mechanism that controls apico-basal polarity in epithelia and neuronal stem cells. This study is the first functional analysis of an atypical protein kinase C isoform using a loss-of-function allele in a genetically tractable organism.
Figures
Figure 1
Structure of the DaPKC locus. (a) The DaPKC gene is located on the right arm of the second chromosome at position 51D. The gene comprises 10 exons (boxes). Translated regions are filled in black and untranslated regions in gray. ATG and TGA mark the translation initiation and termination codons, respectively. In the lethal P-element insertion line l(2)k06403 the PlacW element (hatched box) is inserted into the third intron, which causes a loss-of-function phenotype. (b) Alignment of DaPKC with aPKC isoforms of mouse, rat, and C. elegans. The DaPKC amino acid sequence (aPKC Dm) shows 68% identity to mouse PKCλ (PKCλ Mm), 63% identity to rat PKCζ (PKCζ Rn) and 58% identity to C. elegans PKC-3 (PKC-3 Ce). Amino acid residues identical in at least two of the four kinases are shown in reversed font. The region comprising the kinase domain is underlined. These sequence data are available from GenBank/EMBL/DDBJ under accession number AF288482.
Figure 2
DaPKC and Baz directly bind to each other. (a) Anti-Baz antibody (COOH-terminal, mouse) was used for immunoprecipitation (IP) of an extract from S2 cells overexpressing Baz. Anti-Nrt antibody was used as a negative control. Immunoprecipitates underwent SDS-PAGE and Western analysis with anti-aPKC antibody C20. The Western blot of the cell lysate used for immunoprecipitation (input) is shown on the right. The single band detected by antibody C20 migrates with a molecular weight of ∼75 kD. (b) DaPKC binds directly to Baz. The structure of Baz and the position of the three PDZ domains are shown schematically at the top. The regions of Baz fused in frame with the GAL4 DNA-binding domain in the bait constructs are indicated by bars below the schematic drawing of Baz. In the column on the right: (+) interaction of the bait constructs with full length DaPKC, and (−) the lack of interaction. Numbering of amino acid residues is according to Kuchinke et al. 1998.
Figure 3
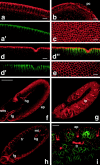
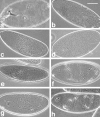
mRNA expression pattern of DaPKC in wild-type embryos. (a) DaPKC mRNA is already present in freshly laid eggs and early embryos before the onset of zygotic transcription and thus must be deposited in the egg during oogenesis. (b) In embryos at the cellular blastoderm stage, DaPKC is expressed in all cells except for the pole cells (arrow). (c) During gastrulation, DaPKC expression is detectable in ectoderm and mesoderm and is elevated in cells that undergo morphogenetic movements; e.g., in the cephalic furrow (cf), in the proctodeal (pr), and the stomodeal (st) invaginations and in the mesoderm (ms). (d) At the extended germ band stage (stage 11), DaPKC is strongly expressed in neuroblasts (nb, arrows) and in most ectodermally derived epithelia, except for the amnioserosa. Note that DaPKC mRNA is highly enriched in the apical cytocortex of several epithelial tissues, including the primordia of the foregut (fg) and hindgut (hg). (e) Higher magnification of a stage 11 embryo showing strong expression of DaPKC in neuroblasts (arrows). (f) Higher magnification of a stage 11 embryo showing apical localization of DaPKC mRNA in foregut (left) and hindgut (right). The apical cell surfaces of fore- and hindgut are marked by arrows and the basal cell surfaces by arrowheads, respectively. Except for f, which is a dorsal view, all embryos are shown in a lateral view. Dorsal is up and anterior to the left. Bar in a–d = 100 μm; bar in e and f = 50 μm.
Figure 4
Expression pattern and subcellular localization of DaPKC protein in wild-type embryos. (a–c) During cellularization (cell cycle 14), DaPKC is already enriched in the apical cytocortex of the newly forming blastoderm cells (a), but is not detectable in the pole cells (pc) (b). Cortical staining of DaPKC is concentrated at cell borders (c). At this stage, plasma membrane polarity has not been fully established yet, as can be seen by the nonpolar distribution of Nrt, an integral membrane protein that becomes later confined to the basolateral membrane (a′). (d–e) In embryos at gastrulation, DaPKC localization is confined to the apicalmost region of the lateral cortex and, at lower levels, to the cortex underlying the free apical surface (d). This localization pattern is complementary to that of Nrt (d′), which is strictly basolateral after completion of cellularization (merged image in d″). In en face views, DaPKC forms a contiguous belt around the apical circumference of each blastoderm cell (e). (f and g) At the extended germ band stage (stage 12), DaPKC is detectable in the apical cortex of all epithelia derived from the ectoderm, except for the amnioserosa. (h and i) Epithelial expression of DaPKC is maintained after germ band retraction (stage 14) and remains confined to the apical cytocortex. sns, stomatogastric nervous system; fg, foregut; hg, hindgut; ep, epidermis; tp, tracheal pits; tr, tracheae; mt, Malpighian tubules. In all panels, DaPKC is in red and Nrt is in green. a and a′, and d, d′, and d″, are double labelings of the same embryo, respectively. f and g show different focal planes of the same embryo. Anterior is to the left and dorsal up in all panels. Bars in a–e and i = 10 μm. Bars in f–h = 100 μm.
Figure 5
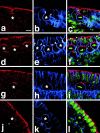
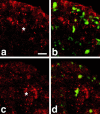
Expression of DaPKC in neuroblasts. Wild-type embryos at the extended germ band stage (stage 10) were triple labeled for DaPKC (red), Nrt (blue), and DNA (YoYo-1, green). (a–c) During delamination of neuroblasts, DaPKC is expressed in the apical stalk that is wedged between adjacent epithelial cells. In prophase (d–f) and metaphase (g–i), DaPKC forms a crescent in the apical cortex of neuroblasts. In anaphase (j–l), DaPKC staining in neuroblasts is strongly reduced but is still detectable in an enlarged region of the apical cortex. No staining is detectable in budding ganglion mother cells (GMCs). Note that Nrt staining is reduced in regions of the plasma membrane where DaPKC is present in the cortex (regions between arrowheads in b and e). Neuroblasts are marked by asterisks and the budding GMCs in j and k by a circle. Merged images of all three stainings are shown in the right column. Apical is up in all panels. Bar = 10 μm.
Figure 6
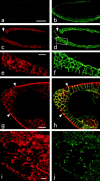
Apical localization of DaPKC depends on Baz. (a–c) DaPKC and Baz are colocalized in epithelia. A wild-type embryo at stage 14 was doubly stained for DaPKC (a, red) and Baz (b, green). Both proteins show extensive colocalization (c). (d–f) Baz is localized apically to the ZA marker Arm. A wild-type embryo at stage 14 was doubly stained for Arm (d, red) and Baz (e, green). In the merged image (f) there is very little overlap and Baz is expressed apically to Arm. The indentations of the epidermis in a–f represent segment boundaries. (g–i) DaPKC localization is lost and Nrt localization is not restricted to the basolateral membrane in baz mutant embryos. In a bazXi106 null embryo at gastrulation, DaPKC is not localized in the cortex (g) and Nrt is present on the whole-cell surface (h), merged image in i. Note also that the blastoderm epithelium is multilayered and cells show a very irregular shape (compare Fig. 4d, Fig. d′, and d″). (j–l) Overexpression of Baz is sufficient for ectopic localization of DaPKC. When overexpressed with the GAL4 system, Baz is not restricted to the apical cortex of epithelial cells and neuroblasts, but becomes localized to ectopic sites in the cortex (k, arrows). Concomitantly, DaPKC is also detectable at ectopic sites in the cortex (j, arrows), where it colocalizes with Baz (l, arrows). Apical is up in all panels. Bars = 10 μm.
Figure 7
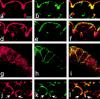
Apical localization of DaPKC in neuroblasts depends on Baz and Insc. (a and b) In a heterozygous inscP49/CyO embryo at stage 10, DaPKC (red) forms apical crescents in metaphase neuroblasts, as in wild type. (c and d) In inscP49 homozygous mutant embryos, apical localization of DaPKC in neuroblasts is lost, while apical localization of DaPKC in the neuroectodermal epithelium is unaffected. (e and f) In bazXi106/+ embryos that are derived from a homozygous mutant germ line (germ line clones with a paternal wild-type copy), asymmetric DaPKC crescents are detectable in neuroblasts, although the neuroectodermal epithelium has almost completely disintegrated. (g and h) In bazXi106/Y embryos derived from germ line clones (baz null), DaPKC localization is completely diffuse and neither cortical nor asymmetric in neuroblasts and epithelia. Note also the abnormal orientation of metaphase plates in d, f, and h. Homozygous mutant inscP49 embryos were identified by the absence of β-galactosidase expression derived from the CyO P[ftz::lacZ] balancer (blue stripe in b). Hemizygous mutant bazXi106/Y embryos were identified by absence of Sex-lethal expression (not shown). DNA was stained with YoYo-1 (green). Apical is up in all panels. Bar = 10 μm.
Figure 8
Cuticle phenotypes of DaPKC and baz mutants are very similar. (a) Wild-type cuticle. (b) Homozygous mutant DaPKCk06403 embryos derived from DaPKCk06403/CyO mothers do not form cuticle because they die before cuticle secretion begins. (c) The same phenotype is observed in embryos from DaPKCk06403/CyO mothers that are transheterozygous for DaPKCk06403 and Df(2R)Jp1 that removes the whole DaPKC coding region. (d) Many bazXi106 null embryos also do not produce cuticle and resemble embryos lacking DaPKC. (e) Embryos from heterozygous DaPKCk06403/CyO mothers that possess a zygotic wild-type allele of DaPKC frequently die with characteristic head defects. (f and g) Embryos derived from C(2)v mothers that are hemizygous for DaPKCk06403 form cuticles with severe head defects and large ventral holes (f) or lack ventral cuticle altogether (g). (h) Zygotic bazXi106 mutants also form cuticles with large ventral holes and head defects, reminiscent of the one shown in f. Anterior is to the left in all panels. Dorsal is up in a–e and g, while f and h are ventral views. Bar = 100 μm.
Figure 10
DaPKC is required for localization of Baz and Insc and for correct orientation of the metaphase plate in neuroblasts. Hemizygous DaPKCk06403 embryos derived from C(2)v mothers were stained for Baz and DNA (a and b) or for Insc and DNA (c and d). Baz staining is strongly reduced and is not localized to the apical cortex in metaphase neuroblasts of DaPKCk06403 hemizygous embryos (a and b), while Insc is still detectable in the cytoplasm but fails to form a tight apical crescent (c and d). Note also that orientation of metaphase plates with respect to the surface of the embryo is abnormal in DaPKCk06403 mutants (b and d). Neuroblasts are marked by asterisks. Apical is up in all panels. b and d are merged images of the Baz and DNA channels (b) or the Insc and DNA channels (d). Bar = 10 μm.
Figure 9
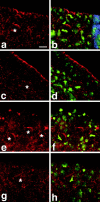
DaPKC mutants show defects in epithelial polarity and morphology. (a and b) Homozygous DaPKCk06403 mutant embryos do not stain with anti-PKCζ antibody C20. A mutant embryo at gastrulation was doubly labeled with anti-PKCζ antibody C20 (a) and anti-Nrt (b). Note the absence of specific staining in a and the multilayering of the blastoderm epithelium in b. (c–f) Homozygous DaPKCk06403 mutant embryos at gastrulation show abnormal localization of Baz and Nrt. Baz (c and e) is not localized to the apical cortex of the blastoderm cells, but instead shows a diffuse, nonpolarized cortical staining. Except for some cells in the outermost cell layer, Nrt (d and f) is localized on the whole plasma membrane and is not restricted to the basolateral membrane, as in wild type. Note the multilayering of the epithelium and the extremely irregular cell shape. e and f are higher magnification views of the boxed area in d. A region devoid of cells due to cell death is marked by an arrow in c and d. (g and h) Embryos derived from heterozygous DaPKCk06403/CyO mothers that carry a zygotic wild-type allele of DaPKC frequently develop characteristic head defects. At the anterior tip (region between arrowheads), the epithelium is multilayered, shows nonpolarized Baz staining (g) and expression of Nrt on the whole cell surface [h, merged channels for Nrt (green) and Baz (red)]. (i and j) Hemizygous DaPKCk06403 embryos derived from C(2)v mothers show loss of Baz (i) and Arm (j) staining in the ventral neuroectoderm. The embryo in g and h is at stage 8 and the embryo in i and j is at stage 10. a–h are optical cross sections and i and j are a view on the ventral surface of the embryo. In all panels, anterior is to the left. Bar in a–d = 100 μm. Bars in e–j = 20 μm.
Similar articles
- Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia.
Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Rolls MM, et al. J Cell Biol. 2003 Dec 8;163(5):1089-98. doi: 10.1083/jcb.200306079. Epub 2003 Dec 1. J Cell Biol. 2003. PMID: 14657233 Free PMC article. - Differential functions of G protein and Baz-aPKC signaling pathways in Drosophila neuroblast asymmetric division.
Izumi Y, Ohta N, Itoh-Furuya A, Fuse N, Matsuzaki F. Izumi Y, et al. J Cell Biol. 2004 Mar 1;164(5):729-38. doi: 10.1083/jcb.200309162. Epub 2004 Feb 23. J Cell Biol. 2004. PMID: 14981094 Free PMC article. - DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila.
Petronczki M, Knoblich JA. Petronczki M, et al. Nat Cell Biol. 2001 Jan;3(1):43-9. doi: 10.1038/35050550. Nat Cell Biol. 2001. PMID: 11146625 - The cell cycle machinery and asymmetric cell division of neural progenitors in the Drosophila embryonic central nervous system.
Chia W, Cai Y, Morin X, Tio M, Udolph G, Yu F, Yang X. Chia W, et al. Novartis Found Symp. 2001;237:139-51; discussion 151-63. doi: 10.1002/0470846666.ch11. Novartis Found Symp. 2001. PMID: 11444041 Review. - Par-3 family proteins in cell polarity & adhesion.
Thompson BJ. Thompson BJ. FEBS J. 2022 Feb;289(3):596-613. doi: 10.1111/febs.15754. Epub 2021 Mar 3. FEBS J. 2022. PMID: 33565714 Free PMC article. Review.
Cited by
- Neural stem cells in Drosophila: molecular genetic mechanisms underlying normal neural proliferation and abnormal brain tumor formation.
Saini N, Reichert H. Saini N, et al. Stem Cells Int. 2012;2012:486169. doi: 10.1155/2012/486169. Epub 2012 Jun 7. Stem Cells Int. 2012. PMID: 22737173 Free PMC article. - Regulation of mitotic spindle orientation: an integrated view.
di Pietro F, Echard A, Morin X. di Pietro F, et al. EMBO Rep. 2016 Aug;17(8):1106-30. doi: 10.15252/embr.201642292. Epub 2016 Jul 18. EMBO Rep. 2016. PMID: 27432284 Free PMC article. Review. - An F1 genetic screen for maternal-effect mutations affecting embryonic pattern formation in Drosophila melanogaster.
Luschnig S, Moussian B, Krauss J, Desjeux I, Perkovic J, Nüsslein-Volhard C. Luschnig S, et al. Genetics. 2004 May;167(1):325-42. doi: 10.1534/genetics.167.1.325. Genetics. 2004. PMID: 15166158 Free PMC article. - Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer.
Neumüller RA, Knoblich JA. Neumüller RA, et al. Genes Dev. 2009 Dec 1;23(23):2675-99. doi: 10.1101/gad.1850809. Genes Dev. 2009. PMID: 19952104 Free PMC article. Review. - Juvenile hormone promotes paracellular transport of yolk proteins via remodeling zonula adherens at tricellular junctions in the follicular epithelium.
Zheng H, Wang N, Yun J, Xu H, Yang J, Zhou S. Zheng H, et al. PLoS Genet. 2022 Jun 27;18(6):e1010292. doi: 10.1371/journal.pgen.1010292. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35759519 Free PMC article.
References
- Adams M.D., Celniker S.E., Holt R.A., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F. The genomic sequence of Drosophila melanogaster . Science. 2000;287:2185–2195. - PubMed
- Baumgartner S., Littleton J.T., Broadie K., Bhat M.A., Harbecke R., Lengyel J.A., Chiquet-Ehrismann R., Prokop A., Bellen H.J. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous