Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons - PubMed (original) (raw)
Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons
J Du et al. J Cell Biol. 2000.
Abstract
Brain-derived neurotrophic factor (BDNF) has been shown to regulate neuronal survival and synaptic plasticity in the central nervous system (CNS) in an activity-dependent manner, but the underlying mechanisms remain unclear. Here we report that the number of BDNF receptor TrkB on the surface of hippocampal neurons can be enhanced by high frequency neuronal activity and synaptic transmission, and this effect is mediated by Ca(2+) influx. Using membrane protein biotinylation as well as receptor binding assays, we show that field electric stimulation increased the number of TrkB on the surface of cultured hippocampal neurons. Immunofluorescence staining suggests that the electric stimulation facilitated the movement of TrkB from intracellular pool to the cell surface, particularly on neuronal processes. The number of surface TrkB was regulated only by high frequency tetanic stimulation, but not by low frequency stimulation. The activity dependent modulation appears to require Ca(2+) influx, since treatment of the neurons with blockers of voltage-gated Ca(2+) channels or NMDA receptors, or removal of extracellular Ca(2+), severely attenuated the effect of electric stimulation. Moreover, inhibition of Ca(2+)/calmodulin-dependent kinase II (CaMKII) significantly reduced the effectiveness of the tetanic stimulation. These findings may help us to understand the role of neuronal activity in neurotrophin function and the mechanism for receptor tyrosine kinase signaling.
Figures
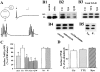
Figure 1
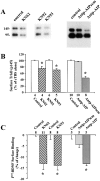
Effects of TBS on BDNF receptors on neurons. Hippocampal neurons were grown in serum free medium for 11–14 d. Two platinum electrodes were positioned on opposite sides of the culture well, and the hippocampal neurons were stimulated with TBS for 60 min in a 37°C incubator in the presence or absence of various inhibitors. After electric stimulation, proteins on the surface of hippocampal neurons were biotinylated, and then examined by Western blot using antibodies against the high affinity receptor TrkB or the low affinity receptor p75. (A) Electrophysiological recording of firing patterns in cultured hippocampal neurons elicited by field electric stimulation. Top traces show that a supra-threshold field stimulation induced a single action potential. Arrow indicates application of a single field electric pulse. Scale: 20 mV and 100 ms. Bottom trace shows an example of action potentials elicited by TBS. 2 out of the 10 bursts are shown. (B) Western blot analysis of cell surface TrkB receptors. Cultures were stimulated with TBS in the presence of indicated agents as follows: TTX (1 μM); kyn (1 mM); Q/M (0.1 mM CNQX plus 80 μM MK801); anisomycin (10 μg/ml); cycloheximide (10 μg/ml); Na+ (50 mM) and K+ (50 mM). (B1) High frequency electric stimulation increases surface expression of TrkB. Both full length (145 kD) and truncated (95 kD) TrkB receptors in stimulated (stim) or unstimulated (unstim) cultures are shown. (B2) Blockade of excitatory synaptic transmission prevents the TBS-induced increase in surface TrkB. The surface TrkB was measured in cultures in the presence or absence of blockers for excitatory transmission, kyn or Q/M. (B3) The total levels of TrkB are not changed by electric stimulation. Hippocampal neurons were stimulated with TBS in the presence or absence of TTX or kyn, harvested by RIPA buffer. Total amount of TrkB were measured directly by Western blot. (B4) TBS-induced increase in surface TrkB does not require protein synthesis. Cultures were stimulated by TBS in the presence or absence of the protein synthesis inhibitor anisomycin or cycloheximide. The surface TrkB were determined by biotinylation. (B5) Simple depolarization induced by high K+ does not change the levels of surface TrkB. Biotinylation was used to determine the levels of surface TrkB in cultures that were treated with 50 mM of either control agent Na+ or the depolarizing agent K+. (C) Summary of the biotinylation experiments for surface TrkB (full length, p145). (Left) The levels of surface TrkB in TBS-stimulated cultures (set as 100%) are compared with unstimulated cultures (unst) and cultures stimulated with TBS in the presence of TTX, kyn, or Q/M; n = 7. (Right) Simple depolarization by high K+ has no effect; n = 4. The surface TrkB levels were determined in cultures that were treated with Na+ (50 mM, set as 100%) and K+ (50 mM). Asterisk indicates statistically different results (P < 0.05, ANOVA followed by post hoc tests). (D) Summary of the biotinylation experiments for surface p75NR. TTX and kyn have no effect on surface p75NR; n = 3. In this and all other bar graph figures, data from a specific experimental condition (e.g., TBS plus TTX) were normalized to the mean in control (TBS stimulation alone) groups. The results in several independent experiments (n) were pooled and averaged, and presented as mean ± SE.
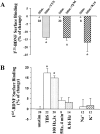
Figure 2
Activity-dependent modulation of the surface binding of BDNF receptors. Cultured hippocampal neurons were incubated with I125-labeled BDNF (5 × 10−11 M) with or without cold BDNF (5 × 10−8 M) for 30 min while stimulated with TBS. Surface binding is defined as acid washable radioactivity at the end of I125-BDNF incubation. The numbers associated with each column represent the total number of experiments. (A) Blockade of neuronal activity or excitatory synaptic transmission prevents the TBS-induced increase in BDNF surface binding. Hippocampal cultures were stimulated with TBS in the presence or absence of TTX, CNQX plus MK801, or kyn. All drug-treated groups were significantly lower than their paired stimulation alone (control) groups, which were set as 0% (P < 0.01, Student's t test). (B) Modulation of BDNF receptor binding by patterned electric stimulation. Percentage of changes is presented. The data in control (no stimulation at all) are set as 0%. TBS and tetanic stimulation (three times, 100 Hz, 1 s every 10 min), but not continuous low frequency stimulation (0.16 Hz) or high K+ (50 mM) stimulation, elicited much higher BDNF receptor binding as compared with control. LTD-inducing stimulus (5 Hz, 4 min) also had no effect. Asterisk indicates statistically different results (P < 0.05, ANOVA followed by post hoc tests).
Figure 3
Role of Ca2+ influx in activity-dependent modulation of cell surface TrkB receptors. Western blot analysis of cell surface TrkB receptors (A) and surface binding of I125-BDNF (B) were performed under conditions that affect Ca2+ influx. TBS was applied in all conditions. Asterisk indicates statistically different results (P < 0.05, ANOVA followed by post hoc tests). (A) Summary of the effect of Ca2+ influx on surface TrkB. Inset shows an example of biotinylation analysis of surface TrkB receptors showing that MK801 and Cd2+ reduce both p145 and p95 TrkB proteins in TBS-stimulated cultures. The amount of full length TrkB (p145) in control conditions (TBS in regular medium) was set as 100%. Ca2+ channels blocked by Cd2+ (0.2 mM) and NMDA receptors blocked by MK801 (80 μM) all inhibited BDNF receptor on the cell surface; n = 7. (B) Summary of the effect of Ca2+ influx on BDNF surface binding. Controls (TBS in regular medium) were set as 0%. In Ca2+-free condition, culture medium was replace and pretreated for 30 min with Ca2+-free DMEM before experiments were performed. The numbers associated with each column represent the total number of experiments.
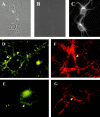
Figure 4
Immunocytochemistry of TrkB on the surface of hippocampal neurons. (A and B) Phase and fluorescence images of neurons stained with an antibody against intracellular domain of TrkB under nonpermeabilizing conditions. (C) Immunofluorescence images of neurons stained with the same antibody permeabilizing conditions. There is no staining in B but good staining in C, indicating that the antibody cannot penetrate inside cells under nonpermeabilizing conditions. (D–G) Immunocytochemistry staining using an antibody against the extracellular domain of TrkB under non-permeabilizing conditions. Hippocampal neurons were stimulated with TBS in the presence (E and G) or absence (D and F) of Cd2+ (0.2 mM) and kyn (1 mM) for 30 min. Cells were fixed with 2% paraformaldehyde, 120 mM sucrose in PBS at room temperature for 3 min, followed by conventional immunofluorescence procedure without any detergent. D and E are conventional immunofluorescence images, and F and G are confocal immunofluorescence images. Arrows indicate surface TrkB stainings on neuronal processes and arrowheads indicate those on cell body. Note that far more surface TrkB receptors are seen in cultures stimulated with TBS alone, especially in neuronal processes. Bar, 5 μm.
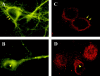
Figure 5
Immunocytochemistry of hippocampal neurons stained by an antibody against the intracellular domain of TrkB under permeabilizing conditions. TBS was applied to the hippocampal neurons in the presence (B and D) or absence (A and C) of Cd2+ and kyn for 30 min. The cultures were then fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.4% Triton X-100 for 60 min, and processed for immunofluorescence staining of TrkB. A and B are conventional immunofluorescence images, and C and D are confocal immunofluorescence images. Arrows indicate cell surface and arrowheads indicate cytoplasmic stainings, respectively. Note that active cells (stimulated by TBS) exhibit TrkB receptors mostly on the cell surface, whereas inactive cells (TBS plus Cd2+ and kyn) show more cytoplasmic staining of TrkB.
Figure 6
Role of CaMKII in activity-dependent modulation of cell surface TrkB receptors. Hippocampal neurons were stimulated with TBS in the presence or absence of KN compounds, or pre-treated with peptides for 3 h before TBS stimulation. Cells were processed either for biotinylation assay for cell surface TrkB receptors (A and B) or for surface binding of I125-BDNF (C). The numbers associated with each column represent the number of experiments. Asterisk indicates statistically different results (P < 0.05, ANOVA followed by post hoc tests). (A) Examples of biotinylation experiments showing that KN62 (10 μM), KN93 (20 μM), and antp-AIP (20 μM), but not KN92 (20 μM) and antp-AIPscm (20 μM), reduce both p145 and p95 TrkB proteins in TBS-stimulated cultures. (B) Summary of the effects of CaMKII inhibitors on surface TrkB (p145). Controls (TBS in regular medium) were set as 100%. (C) Summary of the effect of CaMKII inhibitors on BDNF surface binding. In both B and C, KN62, KN93, and antp-AIP were all effective, whereas KN92 and antp-AIPscm were not.
Similar articles
- The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons.
Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM. Jiang X, et al. J Neurochem. 2005 Aug;94(3):713-22. doi: 10.1111/j.1471-4159.2005.03200.x. Epub 2005 Jul 5. J Neurochem. 2005. PMID: 16000165 - Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx.
Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Du J, et al. J Cell Biol. 2003 Oct 27;163(2):385-95. doi: 10.1083/jcb.200305134. J Cell Biol. 2003. PMID: 14581459 Free PMC article. - Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons.
Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Caldeira MV, et al. J Biol Chem. 2007 Apr 27;282(17):12619-28. doi: 10.1074/jbc.M700607200. Epub 2007 Mar 2. J Biol Chem. 2007. PMID: 17337442 - BDNF-induced local protein synthesis and synaptic plasticity.
Leal G, Comprido D, Duarte CB. Leal G, et al. Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review. - Regulation of TrkB cell surface expression-a mechanism for modulation of neuronal responsiveness to brain-derived neurotrophic factor.
Andreska T, Lüningschrör P, Sendtner M. Andreska T, et al. Cell Tissue Res. 2020 Oct;382(1):5-14. doi: 10.1007/s00441-020-03224-7. Epub 2020 Jun 15. Cell Tissue Res. 2020. PMID: 32556728 Free PMC article. Review.
Cited by
- Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function.
Salinas PC. Salinas PC. Cold Spring Harb Perspect Biol. 2012 Feb 1;4(2):a008003. doi: 10.1101/cshperspect.a008003. Cold Spring Harb Perspect Biol. 2012. PMID: 22300976 Free PMC article. Review. - Consecutive Treatment with Brain-Derived Neurotrophic Factor and Electrical Stimulation Has a Protective Effect on Primary Auditory Neurons.
Scheper V, Seidel-Effenberg I, Lenarz T, Stöver T, Paasche G. Scheper V, et al. Brain Sci. 2020 Aug 15;10(8):559. doi: 10.3390/brainsci10080559. Brain Sci. 2020. PMID: 32824176 Free PMC article. - Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite.
Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Matsuda N, et al. J Neurosci. 2009 Nov 11;29(45):14185-98. doi: 10.1523/JNEUROSCI.1863-09.2009. J Neurosci. 2009. PMID: 19906967 Free PMC article. - Is the tyrosine kinase B receptor a target for preventing epilepsy?
Swanwick CC, Kapur J. Swanwick CC, et al. Epilepsy Curr. 2005 Jan-Feb;5(1):7-10. doi: 10.1111/j.1535-7597.2005.05102.x. Epilepsy Curr. 2005. PMID: 16059446 Free PMC article. No abstract available. - Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons.
Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, Fallon JB, Wallace GG, Shepherd RK, Clark GM, O'Leary SJ. Richardson RT, et al. Biomaterials. 2009 May;30(13):2614-24. doi: 10.1016/j.biomaterials.2009.01.015. Epub 2009 Jan 29. Biomaterials. 2009. PMID: 19178943 Free PMC article.
References
- Berninger B., Poo M.-m. Fast actions of neurotrophic factors. Curr. Opin. Neurobiol. 1996;6:324–330. - PubMed
- Bito H., Deisseroth K., Tsien R.W. CREB phosphorylation and dephosphorylationa Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. - PubMed
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr. Opin. Neurobiol. 1996;6:119–126. - PubMed
- Boulanger L., Poo M.m. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat. Neurosci. 1999;2:346–351. - PubMed
- Cabelli R.J., Horn A., Shatz C.J. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous