Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration - PubMed (original) (raw)
Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration
R V Bhat et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Inactivation of glycogen synthase kinase-3beta (GSK3beta) by S(9) phosphorylation is implicated in mechanisms of neuronal survival. Phosphorylation of a distinct site, Y(216), on GSK3beta is necessary for its activity; however, whether this site can be regulated in cells is unknown. Therefore we examined the regulation of Y(216) phosphorylation on GSK3beta in models of neurodegeneration. Nerve growth factor withdrawal from differentiated PC12 cells and staurosporine treatment of SH-SY5Y cells led to increased phosphorylation at Y(216), GSK3beta activity, and cell death. Lithium and insulin, agents that lead to inhibition of GSK3beta and adenoviral-mediated transduction of dominant negative GSK3beta constructs, prevented cell death by the proapoptotic stimuli. Inhibitors induced S(9) phosphorylation and inactivation of GSK3beta but did not affect Y(216) phosphorylation, suggesting that S(9) phosphorylation is sufficient to override GSK3beta activation by Y(216) phosphorylation. Under the conditions examined, increased Y(216) phosphorylation on GSK3beta was not an autophosphorylation response. In resting cells, Y(216) phosphorylation was restricted to GSK3beta present at focal adhesion sites. However, after staurosporine, a dramatic alteration in the immunolocalization pattern was observed, and Y(216)-phosphorylated GSK3beta selectively increased within the nucleus. In rats, Y(216) phosphorylation was increased in degenerating cortical neurons induced by ischemia. Taken together, these results suggest that Y(216) phosphorylation of GSK3beta represents an important mechanism by which cellular insults can lead to neuronal death.
Figures
Figure 1
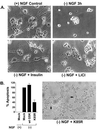
Regulation of Y216 phosphorylation by NGF withdrawal in PC12 cells. (A) Quantification of NGF withdrawal induced apoptosis in PC12 cells by using a DNA fragmentation assay (mean ± SEM, n = 3). (B) GSK3β activity was measured by immunoprecipitation followed by a kinase assay. Cells treated with 10 mM lithium chloride (Li) or 1 μM insulin (Ins) had decreased GSK3β activity. (C) (Left) Western blot showing that the phospho-Y216 specific GSK3β antibody recognizes active rhGSK3β (from Sf9 cells) but not rhGSK3β treated with alkaline phosphatase. (Right) Western blot reprobed with an antibody to total GSK3β. (D) Blot showing increased Y216 phosphorylation after NGF withdrawal (Top) but negligible changes at the S9 phosphorylation site (Middle). (Bottom) Total levels of GSK3β. (E) Western blots showing increased GSK3β phosphorylation on S9 by lithium and insulin, but no significant reduction in Y216 phosphorylation (F), assessed at 3 h after NGF withdrawal. (F Far Right) Immunoprecipitation by anti-HA antibody and Western blot showing Y216 phosphorylation of wt GSK3β-HA and K85R-GSK3β-HA after NGF withdrawal (3 h).
Figure 2
GSK3β inhibition prevents NGF- withdrawal-induced apoptosis in PC12 cells. (A) Photomicrographs of differentiated PC12 cells undergoing apoptosis as a result of NGF withdrawal (Top Left). Lithium (10 mM) pretreatment or addition of insulin (1 μM) prevented apoptosis and maintained relative intact cell morphology. (B) (Left) Effects of adenoviral-mediated transduction of mock (empty vector), wt GSK3β, or dominant negative K85R-GSK3β on apoptosis in PC12 cells. Apoptosis was measured by using the DNA fragmentation assay. K85R-GSK3β protected against NGF withdrawal-induced apoptosis. (Right) Photomicrograph of PC12 cells infected with K85R-GSK3β showing intact neurites (arrows) after withdrawal of NGF.
Figure 3
Regulation of Y216 phosphorylation by staurosporine in SH-SY5Y cells. (A) (Top) Apoptosis was quantified in SH-SY5Y cells treated with staurosporine (0.1 μM) by using the DNA fragmentation assay (mean ± SEM, n = 3). (Middle) Western blot showing an increase in Y216 phosphorylation after staurosporine treatment. (Bottom) Total levels of GSK3β in the samples (B). Representative photomicrograph of SH-SY5Y cells infected with dominant negative K85R-GSK3β after 1 h of staurosporine. Infected cell (arrow) exhibited intact nuclear morphology. (C) Immunoprecipitation and Western blot examining the Y216 phosphorylation of wt GSK3β-HA and K85R-GSK3β-HA expressed in cells treated with staurosporine (1 h). Immunoprecipitation was accomplished by using an HA antibody followed by immunoblotting with the phospho Y216 antibody.
Figure 4
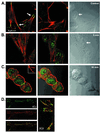
Subcellular localization of phosphorylated Y216 GSK3β. Confocal photomicrographs: phosphorylated Y216 GSK3β (green), actin (red), colocalization (yellow) (A). (Left) Normal SH-SY5Y cells showing colocalization (arrow) of phosphorylated Y216 GSK3β and actin at focal adhesion sites. (Center) Minimal phosphorylated Y216 GSK3β (green) in the nucleus. (Right) Bright-field Nomarski photomicrograph of the cells showing nucleus (arrow). (B) Staurosporine, 5 min. (Left) Lack of phosphorylated Y216 GSK3β (green) and actin (red) colocalization at focal adhesion sites. (Center) Same as_Left_, at the level of the nucleus showing increase in phospho-Y216 immunofluorescence. (Right) Bright-field photomicrograph, arrow points to nucleus. (C) Staurosporine, 30 min. (Left) Lack of phosphorylated Y216 GSK3β (green) and actin (red) colocalization at focal adhesion sites (Inset) but showing increase in Y216 immunofluorescence at the nuclear level. (Center) Overlay with the Nomarski photomicrograph showing the nuclear localization of phosphorylated Y216 GSK3β (green). (Right) Bright-field Nomarski photomicrograph. (D) Untreated SH-SY5Y cells. Y216 GSK3β (green) colocalizes (yellow) with vincullin (red). (Right) High-power photomicrograph of the colocalized proteins.
Figure 5
Ischemia induces Y216 phosphorylation on GSK3β. (A) Western blot showing the increase in Y216 phosphorylation on GSK3β in the ipsilateral cortex (I) when compared with the contralateral cortex (C) at 3 and 6 h after MCAO. Total GSK3β in the tissue samples is shown in the lower Western blot. (B) Representative photomicrographs showing an increase in phosphorylated Y216 immunostaining in neurons in the ipsilateral (ipsi) vs. contralateral (contra) parietal cortex 6 h after MCAO.
Similar articles
- Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2.
Hartigan JA, Xiong WC, Johnson GV. Hartigan JA, et al. Biochem Biophys Res Commun. 2001 Jun 8;284(2):485-9. doi: 10.1006/bbrc.2001.4986. Biochem Biophys Res Commun. 2001. PMID: 11394906 - Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3beta pathway. Implications for neuroprotection.
Hashimoto M, Sagara Y, Langford D, Everall IP, Mallory M, Everson A, Digicaylioglu M, Masliah E. Hashimoto M, et al. J Biol Chem. 2002 Sep 6;277(36):32985-91. doi: 10.1074/jbc.M202803200. Epub 2002 Jul 2. J Biol Chem. 2002. PMID: 12095987 - Mood stabilizers, glycogen synthase kinase-3beta and cell survival.
Jope RS, Bijur GN. Jope RS, et al. Mol Psychiatry. 2002;7 Suppl 1:S35-45. doi: 10.1038/sj.mp.4001017. Mol Psychiatry. 2002. PMID: 11986994 Review. - The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling.
Grimes CA, Jope RS. Grimes CA, et al. Prog Neurobiol. 2001 Nov;65(4):391-426. doi: 10.1016/s0301-0082(01)00011-9. Prog Neurobiol. 2001. PMID: 11527574 Review.
Cited by
- Proteomic Analysis Reveals Physiological Activities of Aβ Peptide for Alzheimer's Disease.
Ai X, Cao Z, Ma Z, Liu Q, Huang W, Sun T, Li J, Yang C. Ai X, et al. Int J Mol Sci. 2024 Jul 30;25(15):8336. doi: 10.3390/ijms25158336. Int J Mol Sci. 2024. PMID: 39125907 Free PMC article. - PI3K/Akt Pathway Contributes to Neurovascular Unit Protection of Xiao-Xu-Ming Decoction against Focal Cerebral Ischemia and Reperfusion Injury in Rats.
Lan R, Xiang J, Zhang Y, Wang GH, Bao J, Li WW, Zhang W, Xu LL, Cai DF. Lan R, et al. Evid Based Complement Alternat Med. 2013;2013:459467. doi: 10.1155/2013/459467. Epub 2013 May 28. Evid Based Complement Alternat Med. 2013. PMID: 23781261 Free PMC article. - GSK3 takes centre stage more than 20 years after its discovery.
Frame S, Cohen P. Frame S, et al. Biochem J. 2001 Oct 1;359(Pt 1):1-16. doi: 10.1042/0264-6021:3590001. Biochem J. 2001. PMID: 11563964 Free PMC article. Review. - Mild chronic cerebral hypoperfusion induces neurovascular dysfunction, triggering peripheral beta-amyloid brain entry and aggregation.
ElAli A, Thériault P, Préfontaine P, Rivest S. ElAli A, et al. Acta Neuropathol Commun. 2013 Nov 13;1:75. doi: 10.1186/2051-5960-1-75. Acta Neuropathol Commun. 2013. PMID: 24252187 Free PMC article. - Characterization of Signaling Pathways Associated with Pancreatic β-cell Adaptive Flexibility in Compensation of Obesity-linked Diabetes in db/db Mice.
Kang T, Boland BB, Jensen P, Alarcon C, Nawrocki A, Grimsby JS, Rhodes CJ, Larsen MR. Kang T, et al. Mol Cell Proteomics. 2020 Jun;19(6):971-993. doi: 10.1074/mcp.RA119.001882. Epub 2020 Apr 7. Mol Cell Proteomics. 2020. PMID: 32265294 Free PMC article.
References
- Raff M C, Barres B A, Burne J, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. - PubMed
- Stefanis L, Burke R E, Greene L A. Curr Opin Neurol. 1997;10:299–305. - PubMed
- Yao R, Cooper G M. Science. 1995;267:2003–2006. - PubMed
- Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R J, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. - PubMed
- Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous