Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury - PubMed (original) (raw)
Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury
J E Springer et al. J Neurosci. 2000.
Abstract
We report here that activation of the caspase-3 apoptotic cascade in spinal cord injury is regulated, in part, by calcineurin-mediated BAD dephosphorylation. BAD, a proapoptotic member of the bcl-2 gene family, is rapidly dephosphorylated after injury, dissociates from 14-3-3 in the cytosol, and translocates to the mitochondria of neurons where it binds to Bcl-x(L). Pretreatment of animals with FK506, a potent inhibitor of calcineurin activity, or MK801, an NMDA glutamate receptor antagonist, blocked BAD dephosphorylation and abolished activation of the caspase-3 apoptotic cascade. These findings extend previous in vitro observations and are the first to implicate the involvement of glutamate-mediated calcineurin activation and BAD dephosphorylation as upstream, premitochondrial signaling events leading to caspase-3 activation in traumatic spinal cord injury.
Figures
Fig. 1.
Representative immunoblots demonstrating that spinal cord injury results in rapid BAD dephosphorylation.A, Immunoblotting experiments demonstrate that spinal cord injury has no effect on overall levels of Bcl-xL, 14-3-3, or calcineurin A over 24 hr.B, BAD levels also are not affected over this period; however, the levels of phosphorylated BAD rapidly decline as early as 30 min after injury. C, Semiquantitative analysis reveals that the levels of phosphorylated BAD are significantly reduced at all time points examined after injury. *p < 0.01 (Scheffe's post hoc analysis).
Fig. 2.
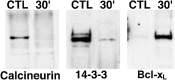
Representative immunoblots demonstrating that spinal cord injury results in the rapid dissociation of BAD from 14-3-3 and calcineurin A followed by binding to Bcl-xL. A phosphorylation state-independent BAD antibody (New England Biolabs) was cross-linked to protein G-Sepharose beads (Sigma). Postmitochondrial supernatant fractions (14-3-3 and calcineurin) or resuspended pellet fractions (Bcl-xL) of control or injured spinal cord were incubated overnight at 4°C with the immobilized BAD antibody. Proteins bound to BAD were eluted and separated by SDS-PAGE, and immunoblotting was used to analyze 14-3-3, calcineurin A and Bcl-xL bound to BAD. The data are representative of experiments from four control laminectomy and four spinal cord-injured animals. CTL, Control;30′, 30 min after injury.
Fig. 3.
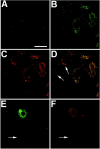
Representative laser-scanned confocal images demonstrating rapid translocation of BAD to the mitochondria and caspase-3 activation after spinal cord injury. Immunofluorescence histochemistry was performed on longitudinal spinal cord sections containing the lesion epicenter. A, Control section demonstrating weak diffuse immunoreactivity for BAD in uninjured gray matter neurons. B, Punctate BAD immunoreactivity in ventral horn neurons in the injury epicenter 1 hr after injury.C, HSP60 immunoreactivity double labeling of the same section as in B. D, Merged images from_B_ and C providing evidence that BAD immunoreactivity is associated with mitochondria. (Note the absence of colocalization in neurons denoted by arrows.) E, F, Double-labeling experiments indicate that caspase-3 activation (F) occurs in cells exhibiting BAD translocation (E). Arrows in_E_ and F denote the absence of BAD or activated capsase-3 staining in a large α motor neuron. These photomicrographs are from 30-μm-thick spinal cord sections corresponding to 2.0 mm rostral to the injury epicenter. Scale bar, 25 μm.
Fig. 4.
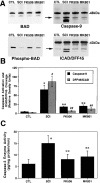
FK506 and MK801 treatments inhibit BAD dephosphorylation and caspase-3 activation. Animals were pretreated with FK506 (10 mg/kg, i.p., 1 hr before injury) or MK801 (0.3 mg/kg, i.p., 30 min before injury), and spinal cords were obtained 1 hr after injury. A, Representative immunoblots demonstrating that FK506 and MK801 treatments inhibited BAD dephosphorylation, caspase-9 activation, and ICAD/DFF45 cleavage. Postmitochondrial supernatant fractions were analyzed by immunoblotting using antibodies to phosphorylation state-independent and -dependent BAD, caspase-9, and ICAD/DFF45. B, Semiquantitative analysis of bands corresponding to activated caspase-9 and caspase-3-like ICAD/DFF45 cleavage (arrows) revealed a significant effect of FK506 and MK801. *p < 0.01 for caspase-9;#p < 0.01 for ICAD/DFF45 (Scheffe's_post hoc_ analysis). C, The caspase fluorogenic assay demonstrated that both FK506 and MK801 significantly reduced caspase-3 enzyme activity after spinal cord injury compared with vehicle treatments. *p < 0.01 compared with laminectomy controls; **p < 0.01 compared with spinal cord injury (Scheffe's post hoc analysis).CTL, Control; SCI, spinal cord injury.
Similar articles
- Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD.
Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Wang HG, et al. Science. 1999 Apr 9;284(5412):339-43. doi: 10.1126/science.284.5412.339. Science. 1999. PMID: 10195903 - Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis.
Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE. Shou Y, et al. Biochem J. 2004 May 1;379(Pt 3):805-13. doi: 10.1042/BJ20031107. Biochem J. 2004. PMID: 14741051 Free PMC article. - The role of calcineurin in amyloid-beta-peptides-mediated cell death.
Cardoso SM, Oliveira CR. Cardoso SM, et al. Brain Res. 2005 Jul 19;1050(1-2):1-7. doi: 10.1016/j.brainres.2005.04.078. Brain Res. 2005. PMID: 15975561 - Seizure-like activity leads to the release of BAD from 14-3-3 protein and cell death in hippocampal neurons in vitro.
Meller R, Schindler CK, Chu XP, Xiong ZG, Cameron JA, Simon RP, Henshall DC. Meller R, et al. Cell Death Differ. 2003 May;10(5):539-47. doi: 10.1038/sj.cdd.4401206. Cell Death Differ. 2003. PMID: 12728252 - Activation of Bcl-2-associated death protein and counter-response of Akt within cell populations during seizure-induced neuronal death.
Henshall DC, Araki T, Schindler CK, Lan JQ, Tiekoter KL, Taki W, Simon RP. Henshall DC, et al. J Neurosci. 2002 Oct 1;22(19):8458-65. doi: 10.1523/JNEUROSCI.22-19-08458.2002. J Neurosci. 2002. PMID: 12351720 Free PMC article.
Cited by
- Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress.
He H, Liu X, Lv L, Liang H, Leng B, Zhao D, Zhang Y, Du Z, Chen X, Li S, Lu Y, Shan H. He H, et al. Cell Death Dis. 2014 Jan 16;5(1):e997. doi: 10.1038/cddis.2013.533. Cell Death Dis. 2014. PMID: 24434520 Free PMC article. - Mechanisms of noise-induced hearing loss indicate multiple methods of prevention.
Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Le Prell CG, et al. Hear Res. 2007 Apr;226(1-2):22-43. doi: 10.1016/j.heares.2006.10.006. Epub 2006 Dec 4. Hear Res. 2007. PMID: 17141991 Free PMC article. Review. - Selective induction of calcineurin activity and signaling by oligomeric amyloid beta.
Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Reese LC, et al. Aging Cell. 2008 Dec;7(6):824-35. doi: 10.1111/j.1474-9726.2008.00434.x. Epub 2008 Sep 8. Aging Cell. 2008. PMID: 18782350 Free PMC article. - The calcineurin inhibitor Ascomicin interferes with the early stage of the epileptogenic process induced by Latrunculin A microperfusion in rat hippocampus.
Freire-Cobo C, Sierra-Paredes G, Freire M, Sierra-Marcuño G. Freire-Cobo C, et al. J Neuroimmune Pharmacol. 2014 Dec;9(5):654-67. doi: 10.1007/s11481-014-9558-9. Epub 2014 Aug 8. J Neuroimmune Pharmacol. 2014. PMID: 25104570 - Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice.
Dineley KT, Hogan D, Zhang WR, Taglialatela G. Dineley KT, et al. Neurobiol Learn Mem. 2007 Sep;88(2):217-24. doi: 10.1016/j.nlm.2007.03.010. Epub 2007 May 22. Neurobiol Learn Mem. 2007. PMID: 17521929 Free PMC article.
References
- Adachi S, Cross AR, Babior BM, Gottlieb RA. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272:21878–21882. - PubMed
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
- Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996;394:321–324. - PubMed
- Bochelen D, Rudin M, Sauter A. Calcineurin inhibitors FK506 and SDZ ASM 981 alleviate the outcome of focal cerebral ischemic/reperfusion injury. J Pharmacol Exp Ther. 1999;288:653–659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials