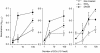In vitro treatment of human transforming growth factor-beta1-treated monocyte-derived dendritic cells with haptens can induce the phenotypic and functional changes similar to epidermal Langerhans cells in the initiation phase of allergic contact sensitivity reaction - PubMed (original) (raw)
In vitro treatment of human transforming growth factor-beta1-treated monocyte-derived dendritic cells with haptens can induce the phenotypic and functional changes similar to epidermal Langerhans cells in the initiation phase of allergic contact sensitivity reaction
S Aiba et al. Immunology. 2000 Sep.
Abstract
Human monocyte-derived dendritic cells (MoDCs) obtained from peripheral blood monocytes (PBMC) cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) can be activated in vitro by a variety of simple chemicals such as haptens and several metals. Recently, it has been demonstrated that transforming growth factor-beta1 (TGF-beta1) can induce further differentiation of MoDCs to the cells that share some characteristics with epidermal Langerhans cells, i.e. they contain Birbeck granules and express E-cadherin. In this study, using such TGF-beta1-treated dendritic cells (TGF-beta1+ DCs), we examined the in vitro effects of representative haptens, i.e. NiCl2 and dinitrochlorobenzene (DNCB), on their phenotypic and functional characteristics, comparing with those reported in vivo in epidermal Langerhans cells during the sensitization phase of a contact sensitivity reaction. Treatment of TGF-beta1+ DCs with NiCl2 increased their expression of the molecules related to antigen presentation such as CD86, major histocompatibility complex class I and class II, and CD83, although weakly, in addition to that of those essential for their migration to the regional lymph nodes, such as CD49e, CD44 and its variant 6, while it down-regulated the expression of the molecules required for homing to the skin and staying in the epidermis, such as cutaneous leucocyte antigen (CLA) and E-cadherin. It also increased the production of tumour necrosis factor-alpha, but not that of IL-1beta or IL-12. DNCB also increased their CD86 expression and down-regulated E-cadherin and CLA, but did not affect other phenotypic changes that were observed in TGF-beta1+ DCs treated with NiCl2. TGF-beta1+ DCs treated with either NiCl2 or DNCB increased their allogeneic T-cell stimulatory function. In addition, reverse transcribed polymerase chain reaction revealed augmented expression of chemokine receptor 7 mRNA by TGF-beta1+ DCs when treated with either NiCl2 or DNCB. Moreover, consistent with this data, TGF-beta1+ DCs treated with these chemicals chemotactically responded to macrophage inflammatory protein-3beta. These data suggest the possibility that TGF-beta1+ DCs present a good in vitro model to study the biology of epidermal Langerhans cells.
Figures
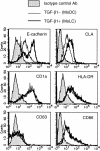
Figure 1
Monocyte-derived DCs treated with TGF-β1 induce E-cadherin and CLA. Peripheral blood CD14+ monocytes were cultured with GM-CSF and IL-4 (MoDCs) or with GM-CSF, IL-4 and TGF-β1 (TGF-β1+ DCs) for 6 days. The surface expression of several phenotypic markers for DCs or LCs were examined by flow cytometry. These are representative data from five different experiments that reproduced a similar staining pattern.
Figure 2
Monocyte-derived DCs treated with TGF-β1 induce LAG expression. The cytospin slides were at first fixed and permeabilized. After blocking with goat serum, the slides were incubated with LAG or non-reactive mouse IgG1 antibody, MOPC 21, and then stained with FITC-conjugated anti-mouse immunoglobulins. The slides were mounted in permafluor containing 0·5 µg/ml propidium iodide, and observed under a confocal microscope. Original magnification: × 200. Inset: original magnification: × 400.
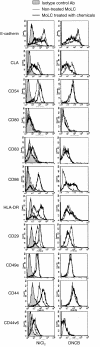
Figure 3
The treatment with NiCl2 and DNCB induced several phenotypic changes on TGF-β1+ DCs. TGF-β1+ DCs differentiated from peripheral blood CD14+ monocytes by GM-CSF, IL-4 and TGF-β1 were treated with 300 µ
m
of NiCl2 or 30 µ
m
of DNCB for 48 hr. These hapten-treated TGF-β1+ DCs were analysed by flow cytometry for their expression of the molecules related to the function of LCs. These are representative data from five different experiments that showed similar results.
Figure 4
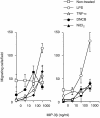
TGF-β1+ DCs treated with haptens increase their allogeneic T-cell stimulatory function. Allogeneic T cells (2 × 105 cells/well) were co-cultured in 96-well flat-bottom microtitre plates with various numbers of TGF-β1+ DCs, which were precultured with 300 µ
m
of NiCl2 or 30 µ
m
of DNCB for 2 days and washed. After 4 days of culture at 37° in a 5% CO2 humidified atmosphere, the cells were pulsed with 10 µ
m
bromodeoxyuridine (BrdU) during the last 16 hr of culture. The allogeneic T-cell stimulation by TGF-β1+ DCs was evaluated by the immunoenzymatic measurement of BrdU uptake by T cells.
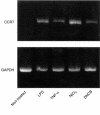
Figure 5
TGF-β1+ DCs treated with haptens increase CCR7 mRNA expression. Total RNA was isolated from TGF-β1+ DCs, which were treated with 10 ng/ml of LPS, 100 ng/ml of TNF-α, 300 µ
m
of NiCl2 or 30 µ
m
of DNCB, for 2 days, and used for RT-PCR with CCR7- and glyceraldehyde-3-phosphate dehydrogenase (G3PDH)-(loading control) specific primers. (a) Non-treated TGF-β1+ DCs; (b) TGF-β1+ DCs treated with 10 ng/ml of LPS; (c) TGF-β1+ DCs treated with 100 ng/ml of TNF-α; (d)TGF-β1+ DCs treated with 300 µ
m
of NiCl2; (e) TGF-β1+ DCs treated with 30 µ
m
of DNCB.
Figure 6
TGF-β1+ DCs treated with haptens respond to MIP-3β. TGF-β1+ DCs, which were precultured with 10 ng/ml of LPS, 100 ng/ml of TNF-α, 300 µ
m
of NiCl2, or 30 µ
m
of DNCB, for 2 days and washed, were assessed by a chemotaxis microchamber technique for their chemotactic responses to MIP-3β; 5 × 104 cells/well in 50 µl of RPMI 1640 medium were applied to the upper wells of the chamber, with a standard 5-µm pore polyvinylpyrrolidone-free polycarbonate filter separating the lower wells. The chamber was incubated at 37° in humidified air with 5% CO2 for 90 min. Then, cells that had migrated to the underside of the filter were counted microscopically at × 200 magnification in five randomly selected fields per well. Each assay was performed in duplicate and the results were expressed as the mean ± SD of migrating cells per field. Shown are two of three experiments with similar results. In these experiments, significant numbers of non-treated TGF-β1+ DCs which did not express CCR7 mRNA and migrated independent of the concentration of MIP-3β, which suggested that their migration was a random migration rather than a chemotactic response.
Similar articles
- TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation.
Ohtani T, Mizuashi M, Nakagawa S, Sasaki Y, Fujimura T, Okuyama R, Aiba S. Ohtani T, et al. Immunology. 2009 Apr;126(4):485-99. doi: 10.1111/j.1365-2567.2008.02919.x. Immunology. 2009. PMID: 19278421 Free PMC article. - Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules.
Aiba S, Terunuma A, Manome H, Tagami H. Aiba S, et al. Eur J Immunol. 1997 Nov;27(11):3031-8. doi: 10.1002/eji.1830271141. Eur J Immunol. 1997. PMID: 9394834 - Simple chemicals can induce maturation and apoptosis of dendritic cells.
Manome H, Aiba S, Tagami H. Manome H, et al. Immunology. 1999 Dec;98(4):481-90. doi: 10.1046/j.1365-2567.1999.00916.x. Immunology. 1999. PMID: 10594678 Free PMC article. - [Cutaneous immune system].
Schmitt D. Schmitt D. C R Seances Soc Biol Fil. 1994;188(3):207-21. C R Seances Soc Biol Fil. 1994. PMID: 7834504 Review. French. - [The Langerhans cell: from in vitro production to use in cellular immunotherapy].
Schmitt D. Schmitt D. J Soc Biol. 2001;195(1):69-74. J Soc Biol. 2001. PMID: 11530504 Review. French.
Cited by
- TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation.
Ohtani T, Mizuashi M, Nakagawa S, Sasaki Y, Fujimura T, Okuyama R, Aiba S. Ohtani T, et al. Immunology. 2009 Apr;126(4):485-99. doi: 10.1111/j.1365-2567.2008.02919.x. Immunology. 2009. PMID: 19278421 Free PMC article. - Study on immune function of dendritic cells in patients with esophageal carcinoma.
Chen SR, Luo YP, Zhang JK, Yang W, Zhen ZC, Chen LX, Zhang W. Chen SR, et al. World J Gastroenterol. 2004 Apr 1;10(7):934-9. doi: 10.3748/wjg.v10.i7.934. World J Gastroenterol. 2004. PMID: 15052669 Free PMC article. - Perspectives on Non-Animal Alternatives for Assessing Sensitization Potential in Allergic Contact Dermatitis.
Sharma NS, Jindal R, Mitra B, Lee S, Li L, Maguire TJ, Schloss R, Yarmush ML. Sharma NS, et al. Cell Mol Bioeng. 2012 Mar;5(1):52-72. doi: 10.1007/s12195-011-0189-4. Cell Mol Bioeng. 2012. PMID: 24741377 Free PMC article. - Construction of a novel necroptosis-related lncRNA signature for prognosis prediction in esophageal cancer.
Liu Y, Hao H, Kang L, Zheng G, Guo X, Li B, Zhao H, Hao H. Liu Y, et al. BMC Gastroenterol. 2022 Jul 15;22(1):345. doi: 10.1186/s12876-022-02421-8. BMC Gastroenterol. 2022. PMID: 35840890 Free PMC article. - p38 Mitogen-Activated Protein Kinase in beryllium-induced dendritic cell activation.
Li L, Huang Z, Gillespie M, Mroz PM, Maier LA. Li L, et al. Hum Immunol. 2014 Dec;75(12):1155-62. doi: 10.1016/j.humimm.2014.10.010. Epub 2014 Oct 22. Hum Immunol. 2014. PMID: 25454621 Free PMC article.
References
- Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791. - PubMed
- Ozawa H, Nakagawa S, Tagami H, Aiba S. Interleukin-1 beta and granulocyte-macrophage colony-stimulating factor mediate Langerhans cell maturation differently. J Invest Dermatol. 1996;106:441. - PubMed
- Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol. 1997;27:3031. - PubMed
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous