Tea2p is a kinesin-like protein required to generate polarized growth in fission yeast - PubMed (original) (raw)
Comparative Study
Tea2p is a kinesin-like protein required to generate polarized growth in fission yeast
H Browning et al. J Cell Biol. 2000.
Abstract
Cytoplasmic microtubules are critical for establishing and maintaining cell shape and polarity. Our investigations of kinesin-like proteins (klps) and morphological mutants in the fission yeast Schizosaccharomyces pombe have identified a kinesin-like gene, tea2(+), that is required for cells to generate proper polarized growth. Cells deleted for this gene are often bent during exponential growth and initiate growth from improper sites as they exit stationary phase. They have a reduced cytoplasmic microtubule network and display severe morphological defects in genetic backgrounds that produce long cells. The tip-specific marker, Tea1p, is mislocalized in both tea2-1 and tea2Delta cells, indicating that Tea2p function is necessary for proper localization of Tea1p. Tea2p is localized to the tips of the cell and in a punctate pattern within the cell, often coincident with the ends of cytoplasmic microtubules. These results suggest that this kinesin promotes microtubule growth, possibly through interactions with the microtubule end, and that it is important for establishing and maintaining polarized growth along the long axis of the cell.
Figures
Figure 1
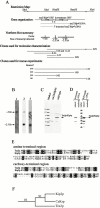
Molecular analysis of tea2 +. (A) Schematic representation of the tea2 + genomic region. The top section is a restriction map (additional HindIII and BamHI sites not shown). The second section illustrates the gene organization with a schematic representation of the ORFs. Arrows indicate the direction of transcription, and wavy lines represent the cDNA clones used in D. The short lines labeled A and B represent the location of the primers used for RT-PCR, which is described in the text. The third section is a summary of the results from the Northern blot analysis shown in B. Lines designated 1, 2, and 3 represent coverage of probes used for the Northern blot analysis, with the size of the transcript detected for each probe written under the line. The fourth section illustrates the clones used for molecular analysis, which are described in the text. Section five shows clones used for rescue experiments. All sections are aligned with the restriction map. (B) Transcriptional analysis of the tea2 + region. Probes designated 1, 2, and 3 in A were hybridized to polyA+ RNA isolated from wild-type cells. (C) Western blot analysis. Protein extracts were obtained from tea2_Δ and wild-type cells in logarithmic growth. The blot was reacted with antibodies to Tea2p. The upper and lower background bands appear with variable intensities from blot to blot. (D) Western blot analysis of cDNA transformants. For expression of the full-length and truncated cDNAs, cells transformed with pREP3X_tea2 +cDNA and pREP3X_tea2_ +ORF were used. The blot was reacted with antibodies to Tea2p. (E) Sequence alignment. Nonmotor regions of Tea2p and Kip2p were aligned using BestFit and displayed using BOXSHADE. Identical aa are highlighted in black, and similar aa are highlighted in grey. (F) Bootstrap analysis. The branch structure shows the Kip2p subfamily of the kinesin superfamily. The numbers illustrate bootstrap values for 100 replicas.
Figure 2
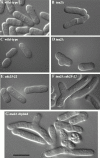
DIC microscopy of (A) wild-type and (B) _tea2_Δ cells grown to mid-log phase. DIC microscopy of (C) wild-type, (D) _tea2_Δ, (E) cdc25-22, and (F) tea2Δ cdc25-22 cells from colonies on plates and (G) tea2Δ diploid cells from liquid culture. Bar: 10 μm.
Figure 3
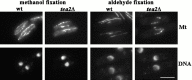
Microtubule staining (Mt) of wild-type and tea2Δ cells grown to mid-log phase and then fixed with methanol or aldehyde and stained with tubulin antibody and DAPI (DNA). Microtubule images are the projection of serial optical sections encompassing the entire depth of the cell. Bar: 5 μ_m._
Figure 4
DIC microscopy of the first and second division out of stationary phase of tea2Δ cells. Cells were grown to saturation in YES medium, placed on a YES agar pad, and examined at the indicated times as the cells exited stationary phase. (Bar: 5 μ_m)._
Figure 5
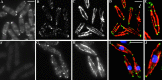
Localization of Tea2p in logarithmic phase cells. (A) Tea2-GFP viewed in live cells. (B–E) tea2-GFP cells fixed and stained with antibodies to (B) GFP, (C) tubulin, (D) merged image, and (E) enlarged, merged image. (D) Cell with arrow has just completed anaphase as exhibited by the postanaphase array of microtubules. (F) tea2Δ cells fixed and stained with antibodies to Tea2p. Wild-type cells fixed and stained with antibodies to (G) Tea2p, (H) tubulin, (I) merged image, and (J) enlarged, merged image. Bar in D corresponds to images B–D; bar in I corresponds to images F–I. Bar: 5 μm.
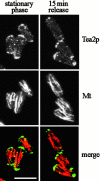
Figure 6
Localization of Tea2p in stationary phase cells and in cells exiting stationary phase. Wild-type cells were grown to stationary phase and then fixed and stained with antibodies to Tea2p (Tea2p) and tubulin (Mt), or were diluted into fresh medium, grown 15 min, and then fixed and stained. Images are the projection of serial optical sections encompassing the entire depth of the cell. Bar: 5 μm.
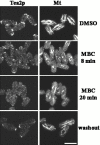
Figure 7
Dependence of Tea2p on microtubules for localization. Cells exiting stationary phase were treated with DMSO (control) or MBC dissolved in DMSO, and then were fixed and stained with antibodies to Tea2p (Tea2p) and tubulin (Mt). MBC was washed out (washout), and the cells were allowed to recover for 18 min, then were fixed and stained. Bar: 5 μm.
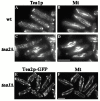
Figure 8
Tea1p localization in tea2Δ cells, and Tea2p-GFP localization in tea1Δ_. (A and B) Wild-type and (C and D)_ tea2Δ cells were grown to logarithmic phase, and then were fixed and stained with antibodies to (A and C) Tea1p and (B and D) tubulin. tea1Δ tea2-GFP cells were stained with antibodies to (E) GFP or (F) tubulin. Bar in D corresponds to A–D; bar in F corresponds to E and F. Bar: 5 μm.
Similar articles
- Roles of fission yeast tea1p in the localization of polarity factors and in organizing the microtubular cytoskeleton.
Behrens R, Nurse P. Behrens R, et al. J Cell Biol. 2002 May 27;157(5):783-93. doi: 10.1083/jcb.200112027. Epub 2002 May 28. J Cell Biol. 2002. PMID: 12034771 Free PMC article. - Tea2p kinesin is involved in spatial microtubule organization by transporting tip1p on microtubules.
Busch KE, Hayles J, Nurse P, Brunner D. Busch KE, et al. Dev Cell. 2004 Jun;6(6):831-43. doi: 10.1016/j.devcel.2004.05.008. Dev Cell. 2004. PMID: 15177031 - Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast.
Glynn JM, Lustig RJ, Berlin A, Chang F. Glynn JM, et al. Curr Biol. 2001 Jun 5;11(11):836-45. doi: 10.1016/s0960-9822(01)00235-4. Curr Biol. 2001. PMID: 11516644 - Cell polarity: a tale of two Ts.
Verde F. Verde F. Curr Biol. 2001 Aug 7;11(15):R600-2. doi: 10.1016/s0960-9822(01)00362-1. Curr Biol. 2001. PMID: 11516965 Review. - Studies in fission yeast on mechanisms of cell division site placement.
Chang F. Chang F. Cell Struct Funct. 2001 Dec;26(6):539-44. doi: 10.1247/csf.26.539. Cell Struct Funct. 2001. PMID: 11942607 Review.
Cited by
- Microtubule-organizing center formation at telomeres induces meiotic telomere clustering.
Yoshida M, Katsuyama S, Tateho K, Nakamura H, Miyoshi J, Ohba T, Matsuhara H, Miki F, Okazaki K, Haraguchi T, Niwa O, Hiraoka Y, Yamamoto A. Yoshida M, et al. J Cell Biol. 2013 Feb 18;200(4):385-95. doi: 10.1083/jcb.201207168. Epub 2013 Feb 11. J Cell Biol. 2013. PMID: 23401002 Free PMC article. - Widespread cotranslational formation of protein complexes.
Duncan CD, Mata J. Duncan CD, et al. PLoS Genet. 2011 Dec;7(12):e1002398. doi: 10.1371/journal.pgen.1002398. Epub 2011 Dec 1. PLoS Genet. 2011. PMID: 22144913 Free PMC article. - The novel fission yeast protein Pal1p interacts with Hip1-related Sla2p/End4p and is involved in cellular morphogenesis.
Ge W, Chew TG, Wachtler V, Naqvi SN, Balasubramanian MK. Ge W, et al. Mol Biol Cell. 2005 Sep;16(9):4124-38. doi: 10.1091/mbc.e04-11-0976. Epub 2005 Jun 22. Mol Biol Cell. 2005. PMID: 15975911 Free PMC article. - Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia.
Bowen JR, Hwang D, Bai X, Roy D, Spiliotis ET. Bowen JR, et al. J Cell Biol. 2011 Jul 25;194(2):187-97. doi: 10.1083/jcb.201102076. J Cell Biol. 2011. PMID: 21788367 Free PMC article. - PKA antagonizes CLASP-dependent microtubule stabilization to re-localize Pom1 and buffer cell size upon glucose limitation.
Kelkar M, Martin SG. Kelkar M, et al. Nat Commun. 2015 Oct 7;6:8445. doi: 10.1038/ncomms9445. Nat Commun. 2015. PMID: 26443240 Free PMC article.
References
- Allen V.W., Kropf D.L. Nuclear rotation and lineage specification in Pelvetia embryos. Development. 1992;115:873–883.
- Bahler J., Wu J.-Q., Longtine M.S., Shah N.G., McKenzie A., III, Steever A.B., Wach A., Philippsen P., Pringle J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe . Yeast. 1998;14:943–951. - PubMed
- Barbet N., Muriel W.J., Carr A.M. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe . Gene. 1992;114:59–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous