Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3beta - PubMed (original) (raw)
Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3beta
O Krylova et al. J Cell Biol. 2000.
Abstract
Dishevelled has been implicated in the regulation of cell fate decisions, cell polarity, and neuronal function. However, the mechanism of Dishevelled action remains poorly understood. Here we examine the cellular localization and function of the mouse Dishevelled protein, DVL-1. Endogenous DVL-1 colocalizes with axonal microtubules and sediments with brain microtubules. Expression of DVL-1 protects stable microtubules from depolymerization by nocodazole in both dividing cells and differentiated neuroblastoma cells. Deletion analyses reveal that the PDZ domain, but not the DEP domain, of DVL-1 is required for microtubule stabilization. The microtubule stabilizing function of DVL-1 is mimicked by lithium-mediated inhibition of glycogen synthase kinase-3beta (GSK-3beta) and blocked by expression of GSK-3beta. These findings suggest that DVL-1, through GSK-3beta, can regulate microtubule dynamics. This new function of DVL-1 in controlling microtubule stability may have important implications for Dishevelled proteins in regulating cell polarity.
Figures
Figure 1
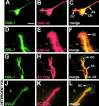
DVL-1 expression in postnatal mouse brain. Brain sections were immunostained for DVL-1 (A–J). The highest level of DVL-1 was detected in neurons of the cerebral cortex, hippocampus, pons, and cerebellum (A–C and I). (A, inset) DVL-1 localization in cell bodies and processes of pyramidal neurons. In P0 cerebellum, DVL-1 is expressed in the EGL and forming IGL (D). At P7, DVL-1 immunoreactivity increases in the EGL (E). At P14, DVL-1 was mainly localized in the granule cell layer (GCL) and in the Purkinje cell (PC) layer (F). This pattern of DVL-1 expression was maintained throughout life (G–I). A low level of DVL-1 expression was detected in the molecular layer (ML) from P21 (G–I). At P21, DVL-1 is mainly localized in granule cells and Purkinje cells cell bodies, as shown at higher magnification (J). No immunoreactivity was detected in the cerebellum of adult Dvl-1 null mutant mice (K). Preincubation of the antibody with DVL-46 peptide completely abolished immunostaining in adult cerebellum (L). AD, adult; DG, dentate gyrus. Bar, 100 μM.
Figure 2
DVL-1 isoforms are developmentally regulated. Western blot analysis of cerebellar extracts from P0, P6, P14, P21, and adult mice reveals changes in the level of three DVL-1 isoforms. DVL-1 antibody recognizes three DVL-1 species of 96 kD (arrow), 88 kD (arrowhead), and 83 kD (asterisk). At birth, the three forms of DVL-1 are present, but the 83-kD isoform is expressed at low levels. During cerebellar maturation, expression of the 83-kD isoform increases, while the two higher molecular weight isoforms decrease. Cerebella samples from Dvl-1 null mutant mice, used as a negative control, reveal the absence of the three DVL-1 isoforms. Expression of DVL-1 in QT-6 cells reveals the presence of the high molecular weight isoforms (DVL-1). The same blot was reprobed with an antibody against extracellular signal-regulated kinase to control for loading.
Figure 3
The subcellular distribution of DVL-1 in maturing cerebellar granule cell neurons. Granule cell cultures were grown for 2 d and stained with antibodies to DVL-1 (A, D, G, and J), GAP-43 (B and E), and acetylated tubulin (H and K). DVL-1 has a punctate distribution in the neuronal cell bodies and along the axon shaft (A–C). High levels of DVL-1 were detected in the central domain of the growth cone (D and F). DVL-1 immunostaining colocalizes with acetylated tubulin along the axon shaft (G–I). In neurons fixed in the presence of detergent, a pool of DVL-1 remains colocalized with acetylated tubulin (J–L). CB, cell body; AS, axon shaft; GC, growth cone. Bar: A–C and G–I, 20 μM; D–F and J–L, 10 μM.
Figure 4
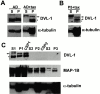
DVL-1 cosediments with stabilized microtubules. (A) In adult brain lysate, the endogenous 83-kD DVL-1 isoform is equally distributed between supernatant (S) and pellet (P) fractions (AD). In the presence of taxol, the 83-kD isoform (asterisk) becomes more abundant in the stabilized MT pellet (P) with low levels of the 96-kD isoform (arrow; AD+tax). The high molecular weight band in the soluble fraction of taxol-treated lysate is not specific. Reprobing for α-tubulin reveals an enrichment of MTs in the pellet fraction from taxol-containing samples. (B) In P5 brain samples treated with taxol, high levels of the 88-kD DVL-1 protein (arrowhead) and low levels of the 96-kD (arrow) and 83-kD (asterisk) DVL-1 proteins are detected in the taxol-MT pellet (P). Low levels of the 96-kD isoform is found in the soluble fraction (S). Reprobing for α-tubulin reveals an enrichment of MTs in the pellet fraction of taxol-treated lysates. (C) DVL-1 cosediments with the cold stable MT pellet from adult brain. High levels of the 83-kD (asterisk) and low levels of the 96-kD (arrow) DVL-1 proteins were found in the soluble (S1) and MT fractions (P1). After a round of depolymerization, DVL-1 is most abundant in the cold-stable MT pellet (CSP1). No DVL-1 was detected with MT fractions in subsequent cycles of polymerization. High levels of MAP-1B cosediment with the cold-stable MT pellet (CSP1), whereas low levels of MAP-1 are found after MT cycling (P2 and P3).
Figure 5
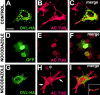
DVL-1 prevents the loss of stable microtubules in nocodazole treated COS-7 cells. COS-7 expressing DVL-HA (A–C and G–I) or GFP (D–F) were examined for the level of MTs (B, E, and H). COS-7 cells expressing DVL-HA (A) have a normal level of acetylated MTs (B and C). COS-7 cells expressing GFP (D–F) or DVL-HA (G–I) were treated with nocodazole to depolymerize MTs. GFP-expressing cells have no acetylated MTs, similar to neighboring untransfected cells (D–F). Expression of DVL-HA prevents MT depolymerization by nocodazole (G–I). Arrows denote MTs resistant to nocodazole in the cell expressing DVL-HA. *Neighboring untransfected cell, which has lost stable MTs. (Inset) Small vesicle-like structures are present on some MTs. Bar, 50 μM.
Figure 6
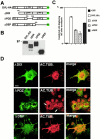
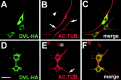
Microtubule stabilization by DVL-1 requires the PDZ domain. (A) Schematic representation of DVL-1 full-length and deletion constructs with the conserved domains. (B) Western analysis using the anti–HA antibody shows that COS-7 cells transfected with the full length and deletion constructs express similar levels of DVL-1 proteins. (C) Quantification of the number of transfected cells containing MTs after nocodazole treatment. The error bars show mean ± SEM (results of at least three experiments). More than 150 cells were counted in each experiment for each deletion construct. (D) COS-7 cells expressing different deletions of DVL-1 were double-immunostained for DVL-HA (green) and acetylated tubulin (red). Cells expressing ΔDIX have some MTs after nocodazole treatment, whereas expression of ΔPDZ confers no protection. Cells expressing ΔDEP have similar levels of MTs as cells expressing full length DVL-1. Arrows indicate the presence of very few MTs in ΔPDZ-expressing cells. Bar, 50 μM.
Figure 7
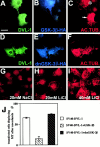
Inhibition of GSK-3β by DVL-1 or lithium stabilizes microtubules. (A–C) COS-7 cells double transfected with DVL-1 and GSK-3β-HA were treated with nocodazole. The cells were fixed and triple-stained with antibodies to DVL-1 (green), HA (blue), and acetylated tubulin (red). Cells expressing DVL-1 and GSK-3β-HA have very low level of acetylated MTs (asterisk), whereas a neighboring cell expressing only DVL-1 (C, arrow) contains MTs after nocodazole treatment. (D–F) Expression of the kinase-dead form of GSK-3β (dnGSK-3β-HA) in DVL-1–expressing cells does not block the ability of DVL-1 to protect MTs against nocodazole treatment. (G) COS-7 cells cultured in control NaCl-containing medium have no stable MTs after nocodazole treatment. (H) Cells treated with 20 mM LiCl have few acetylated MTs after nocodazole treatment, whereas in the presence of 40 mM LiCl (I), a larger proportion of acetylated MTs is present in nocodazole-treated cells. (J) Graph shows the percentage of cells containing MTs after nocodazole treatment. Note that expression of dnGSK-3β and DVL-1 results in a small but significant increase in the number of cells containing MTs (65.6% and 74.4%; t test, P < 0.005). Values are mean ± SEM from three separate experiments. Bar, 50 μM.
Figure 8
DVL-1 stabilizes axonal microtubules. (A–C) Differentiated NB2a cells expressing DVL-HA have higher levels of acetylated MTs (arrows) than untransfected neighboring cells (arrowhead). (D–F) Neurons expressing DVL-HA have acetylated MTs after nocodazole treatment (arrow), whereas untransfected cells have no acetylated MTs (asterisk). Bar, 25 μM.
Similar articles
- A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules.
Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. Ciani L, et al. J Cell Biol. 2004 Jan 19;164(2):243-53. doi: 10.1083/jcb.200309096. J Cell Biol. 2004. PMID: 14734535 Free PMC article. - c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability.
Ciani L, Salinas PC. Ciani L, et al. BMC Cell Biol. 2007 Jul 3;8:27. doi: 10.1186/1471-2121-8-27. BMC Cell Biol. 2007. PMID: 17608927 Free PMC article. - Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription.
Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Smalley MJ, et al. EMBO J. 1999 May 17;18(10):2823-35. doi: 10.1093/emboj/18.10.2823. EMBO J. 1999. PMID: 10329628 Free PMC article. - New steps in the Wnt/beta-catenin signal transduction pathway.
Sakanaka C, Sun TQ, Williams LT. Sakanaka C, et al. Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review. - Retrograde signalling at the synapse: a role for Wnt proteins.
Salinas PC. Salinas PC. Biochem Soc Trans. 2005 Dec;33(Pt 6):1295-8. doi: 10.1042/BST0331295. Biochem Soc Trans. 2005. PMID: 16246102 Review.
Cited by
- Planar cell polarity induces local microtubule bundling for coordinated ciliary beating.
Nakayama S, Yano T, Namba T, Konishi S, Takagishi M, Herawati E, Nishida T, Imoto Y, Ishihara S, Takahashi M, Furuta K, Oiwa K, Tamura A, Tsukita S. Nakayama S, et al. J Cell Biol. 2021 Jul 5;220(7):e202010034. doi: 10.1083/jcb.202010034. J Cell Biol. 2021. PMID: 33929515 Free PMC article. - Neurodevelopment in schizophrenia: the role of the wnt pathways.
Panaccione I, Napoletano F, Forte AM, Kotzalidis GD, Del Casale A, Rapinesi C, Brugnoli C, Serata D, Caccia F, Cuomo I, Ambrosi E, Simonetti A, Savoja V, De Chiara L, Danese E, Manfredi G, Janiri D, Motolese M, Nicoletti F, Girardi P, Sani G. Panaccione I, et al. Curr Neuropharmacol. 2013 Sep;11(5):535-58. doi: 10.2174/1570159X113119990037. Curr Neuropharmacol. 2013. PMID: 24403877 Free PMC article. - Wnt signalling tunes neurotransmitter release by directly targeting Synaptotagmin-1.
Ciani L, Marzo A, Boyle K, Stamatakou E, Lopes DM, Anane D, McLeod F, Rosso SB, Gibb A, Salinas PC. Ciani L, et al. Nat Commun. 2015 Sep 24;6:8302. doi: 10.1038/ncomms9302. Nat Commun. 2015. PMID: 26400647 Free PMC article. - Dishevelled proteins are associated with olfactory sensory neuron presynaptic terminals.
Rodriguez-Gil DJ, Hu W, Greer CA. Rodriguez-Gil DJ, et al. PLoS One. 2013;8(2):e56561. doi: 10.1371/journal.pone.0056561. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437169 Free PMC article. - Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase.
Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, Pollard C, Killick R, Iqbal T, Raymond L, Varndell I, Sheppard P, Makoff A, Gower E, Soden PE, Lewis P, Murphy M, Golde TE, Rupniak HT, Anderton BH, Lovestone S. Mudher A, et al. J Neurosci. 2001 Jul 15;21(14):4987-95. doi: 10.1523/JNEUROSCI.21-14-04987.2001. J Neurosci. 2001. PMID: 11438574 Free PMC article.
References
- Adler P.N. The genetic control of tissue polarity in Drosophila . Bioessays. 1992;14:735–741. - PubMed
- Arias A.M., Brown A.M., Brennan K. Wnt signallingpathway or network? Curr. Opin. Genet. Dev. 1999;9:447–454. - PubMed
- Atack J.R., Broughton H.B., Pollack S.J. Inositol monophosphatasea putative target for Li+ in the treatment of bipolar disorder. Trends Neurosci. 1995;18:343–349. - PubMed
- Axelrod J.D., Matsuno K., Artavanis-Tsakonas S., Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271:1826–1832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources