VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain - PubMed (original) (raw)
VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain
Z G Zhang et al. J Clin Invest. 2000 Oct.
Abstract
VEGF is a secreted mitogen associated with angiogenesis and is also a potent vascular permeability factor. The biological role of VEGF in the ischemic brain remains unknown. This study was undertaken to investigate whether VEGF enhances cerebral microvascular perfusion and increases blood-brain barrier (BBB) leakage in the ischemic brain. Using magnetic resonance imaging (MRI), three-dimensional laser-scanning confocal microscope, and functional neurological tests, we measured the effects of administrating recombinant human VEGF(165) (rhVEGF(165)) on angiogenesis, functional neurological outcome, and BBB leakage in a rat model of focal cerebral embolic ischemia. Late (48 hours) administration of rhVEGF(165) to the ischemic rats enhanced angiogenesis in the ischemic penumbra and significantly improved neurological recovery. However, early postischemic (1 hour) administration of rhVEGF(165) to ischemic rats significantly increased BBB leakage, hemorrhagic transformation, and ischemic lesions. Administration of rhVEGF(165) to ischemic rats did not change BBB leakage and cerebral plasma perfusion in the contralateral hemisphere. Our results indicate that VEGF can markedly enhance angiogenesis in the ischemic brain and reduce neurological deficits during stroke recovery and that inhibition of VEGF at the acute stage of stroke may reduce the BBB permeability and the risk of hemorrhagic transformation after focal cerebral ischemia.
Figures
Figure 1
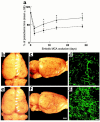
Composite images (through stacks of 14 optical sections of 5-μm thickness) of cerebral microvascular plasma perfusion obtained from the ischemic penumbra of the cortex 9 days after MCA occlusion. Late treatment with rhVEGF165 enhances cerebral microvascular plasma perfusion in the penumbra (b) compared with the control rat (a). Treatment with rhVEGF165 did not alter cerebral microvascular plasma perfusion in the contralateral hemisphere (d), compared with the contralateral hemisphere in the control rat (c). Bar, 100 μm. Quantitative data analyzed by the MIRAGE program (g) show a significant increase of plasma perfusion in vessels within 3 μm of diameters in the rhVEGF165–treated group (filled bars; n = 5) compared with the saline-treated group (open bars; n = 6; A_P_ < 0.05). Higher-magnification images show that cerebral microvessels in the ipsilateral hemisphere had an irregular pattern of tortuosity (e) compared with the vessels in the contralateral hemisphere (f). Dimension of the composite image is 260.6 × 260.6 × 20 μm3. (h) The percentage of vessels with different diameters in the entire vessels perfused by FITC-dextran. The number of vessels with diameters within 2 μm is significantly (P < 0.01) higher in the ipsilateral hemisphere (filled squares; n = 5) than in the contralateral hemisphere (open squares; n = 5).
Figure 2
(a) The percentage of time that rats persisted on the rotarod after ischemia compared with the preischemic value as a function of time of stroke. Rotarod test was performed at 2, 7, 14, and 28 days after stroke on the ischemic rats treated with rhVEGF165 or saline initiated at 48 hours after ischemia. Treatment with rhVEGF 165 (triangles; n = 7) significantly (A_P_ < 0.05 or B_P_ < 0.01) increases time spent on the rotarod compared with the saline-treated group (circles; n = 10). Cerebral vessels and FITC-dextran–perfused cerebral microvessels from the saline-treated rat (b–d) and the rhVEGF165-treated rat (e–g) 28 days after the right MCA occlusion. Dorsal view of the right and left hemisphere vessels (b and e), a lateral view of the right hemisphere vessels (c and f), and FITC-dextran–perfused cerebral microvessels in the penumbra of the cortex (d and g). More vessels in the ipsilateral ischemic border zone (e, arrows, and f) and more FITC-dextran–perfused vessels (g) were observed in the rhVEGF165-treated rat compared with that in the saline-treated rat (b–d). Bar = 2 mm in f for b, c, e, and f; bar = 200 μm in g for d and g. Rats were decapitated one minute after injection of FITC-dextran and their brains were rapidly removed from the severed heads and digitized at 8× magnification (for panels b, c, e, and f) using an MCID image analysis system (Imaging Research, St. Catherines, Canada). After digitization the brains were placed in 4% paraformaldehyde at 4°C for 48 hours (for panels d and g).
Figure 3
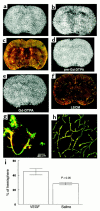
Gd-DTPA contrast-enhanced MRI images of ischemic brains treated with rhVEGF165 at 1 hour after MCA occlusion (a and b) or 0.9% saline (d and e) and corresponding images of LSCM (c and f). Postcontrast images obtained at 30 minutes after injection of Gd-DTPA agent show the hyperintense areas in the ipsilateral MCA-supplied cortex and subcortex of a rat treated with rhVEGF165 (b) and in the ipsilateral MCA-supplied subcortex of a rat treated with 0.9% saline (e), which are not seen on the precontrast images (a and d). Composite images of LSCM (80 μm thick) from the same locations exhibit extravasation of Evans blue and FITC-dextran in the cortex and subcortex from the rat treated with rhVEGF165 (c) and extravasation of Evans blue in the subcortex from the rat treated with saline (f) and sacrificed 6 hours after MCA occlusion. High-magnification images of LSCM from the rat treated with rhVEGF165 show that the extravasation of Evans blue (g, red) and FITC-dextran (g, green) was present in capillaries with nonplasma and low-plasma perfusion in the ipsilateral hemisphere (g) and that cerebral microvessels in the contralateral hemisphere were well perfused with both dyes and did not exhibit any extravasation of fluorescent dyes (h). Bar, 40 μm for g and h. Hyperintense areas on Gd-DTPA-enhanced MRI are significantly (P < 0.05) larger in rhVEGF165–treated rats (n = 5) than the saline-treated rats (n = 4) (i). Hyperintense areas were measured on Gd-DTPA–enhanced images obtained at 30 minutes after injection of Gd-DTPA agent, which was injected after 4 hours of infusion of rhVEGF165 or saline.
Figure 4
Increase in the BBB leakage and hemorrhagic transformation. Large hyperintense areas on Gd-DTPA–enhanced MRI were observed 30 minutes after injection of Gd-DTPA in a rat treated with rhVEGF165 (b), compared with the precontrast image (a). A coronal section corresponding to the MRI section obtained at 24 hours after a stroke shows extensive Evans blue leakage and hemorrhage (c and d). Bar, 1 mm in d.
Figure 5
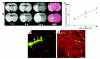
Evolution of the ischemic lesion from 1 hour to 24 hours after MCA occlusion in a rat treated with 0.9% saline (a) or rhVEGF165 (b). Although areas of hyperintensity on the MRI were relatively similar at 1 hour of MCA occlusion in saline-treated (a) or rhVEGF165–treated (b) rat, an area of hyperintensity on MRI was larger at 6 hours and 24 hours of ischemia in the rhVEGF-treated (b) rat compared with an area of hyperintensity at the same time points in the saline-treated rat (a). Differences of ischemic lesions observed on MRI between these two rats are confirmed on H&E-stained coronal sections obtained at 24 hours of ischemia (H&E) (a and b). Quantitative analysis of the ischemic area as a percentage of hemisphere measured on DWI at 6 hours and 24 hours of MCA occlusion (c) shows that ischemic areas are significantly (A_P_ < 0.05) larger in rats treated with rhVEGF165 (solid line, n = 6) than in the control rats (dotted line, n = 7) at 6 hours and 24 hours of embolic MCA occlusion. Composite images (135 × 135 × 20 μm3) of microvessels (FITC-dextran, green) and MAP2 immunoreactivity (Cy5, red) show much less MAP2 immunoreactivity (red) in low plasma-perfused areas with extravasation of FITC-dextran (green) in the ipsilateral hemisphere (d) compared with the homologous tissue in the contralateral hemisphere (e) in a rat treated with rhVEGF165 (1 hour) and sacrificed at 6 hours after MCA occlusion. Bar, 20 μm for d and e.
Figure 6
Late (48 hours) administration of rhVEGF165 to ischemic rats did not result in increases in BBB leakage. Postcontrast images (b) obtained at 30 minutes after injection of the Gd-DTPA agent did not show an increase in areas of hyperintensity compared with the precontrast image (a) in a rat treated with rhVEGF165 at 48 hours of MCA occlusion. Non- and low cerebral microvascular plasma perfusion was detected in the ipsilateral MCA-supplied territory on a composite image of LSCM (80 μm thickness) (c). However, extravasation of Evans blue and FITC-dextran was not detected (c). Images of LSCM were obtained immediately after the last Gd-DTPA–enhanced MRI measurements.
Figure 7
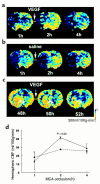
CBF maps of coronal sections measured by perfusion-weighted MRIs. Images of CBF were obtained before, during, and at the end of infusion of rhVEGF165 (a) and saline (b) initiated at 1 hour after embolization. Increase of CBF was observed in the ischemic lesion during infusion of rhVEGF165 (2 hours) (a). The increase in CBF in the rhVEGF165–treated rats (triangles; n = 6) (d) was significant (P < 0.05) compared with that in the saline-treated animals (circles; n = 6). Images of CBF were obtained before, during, and at the end of infusion of rhVEGF165 initiated at 48 hours after embolization (c). Hyperemia was evident in the ischemic lesion before infusion of rhVEGF165 (48 hours) (c). Infusion of rhVEGF165 decreases hyperemic areas in the ischemic lesion (50 hours and 52 hours) (c).
Similar articles
- Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia.
Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Zhang ZG, et al. J Cereb Blood Flow Metab. 2002 Apr;22(4):379-92. doi: 10.1097/00004647-200204000-00002. J Cereb Blood Flow Metab. 2002. PMID: 11919509 - VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia.
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. Sun Y, et al. J Clin Invest. 2003 Jun;111(12):1843-51. doi: 10.1172/JCI17977. J Clin Invest. 2003. PMID: 12813020 Free PMC article. - Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia.
Zhang Z, Chopp M. Zhang Z, et al. Trends Cardiovasc Med. 2002 Feb;12(2):62-6. doi: 10.1016/s1050-1738(01)00149-9. Trends Cardiovasc Med. 2002. PMID: 11852252 Review. - Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain.
Krum JM, Mani N, Rosenstein JM. Krum JM, et al. Neuroscience. 2002;110(4):589-604. doi: 10.1016/s0306-4522(01)00615-7. Neuroscience. 2002. PMID: 11934468 - The Janus Face of VEGF in Stroke.
Geiseler SJ, Morland C. Geiseler SJ, et al. Int J Mol Sci. 2018 May 4;19(5):1362. doi: 10.3390/ijms19051362. Int J Mol Sci. 2018. PMID: 29734653 Free PMC article. Review.
Cited by
- Leukocyte TNFR1 and TNFR2 Expression Contributes to the Peripheral Immune Response in Cases with Ischemic Stroke.
Hansen RB, Laursen CCH, Nawaz N, Madsen JS, Nielsen HH, Kruuse C, Møller A, Degn M, Lambertsen KL. Hansen RB, et al. Cells. 2021 Apr 9;10(4):861. doi: 10.3390/cells10040861. Cells. 2021. PMID: 33918875 Free PMC article. - Vasoregulatory Autoantibodies and Clinical Outcome After Ischemic Stroke-PROSCIS-B.
Liman TG, Siegerink B, Piper S, Catar R, Moll G, Riemekasten G, Heidecke H, Heuschmann PU, Elkind MSV, Dragun D, Endres M. Liman TG, et al. J Am Heart Assoc. 2023 Dec 5;12(23):e032441. doi: 10.1161/JAHA.123.032441. Epub 2023 Nov 28. J Am Heart Assoc. 2023. PMID: 38014691 Free PMC article. - Recovery of fine motor performance after ischemic damage to motor cortex is facilitated by cell therapy in the rhesus monkey.
Moore TL, Pessina MA, Finklestein SP, Kramer BC, Killiany RJ, Rosene DL. Moore TL, et al. Somatosens Mot Res. 2013 Dec;30(4):185-96. doi: 10.3109/08990220.2013.790806. Epub 2013 Jun 12. Somatosens Mot Res. 2013. PMID: 23758412 Free PMC article. - Clinical significance of plasma VEGF value in ischemic stroke - research for biomarkers in ischemic stroke (REBIOS) study.
Matsuo R, Ago T, Kamouchi M, Kuroda J, Kuwashiro T, Hata J, Sugimori H, Fukuda K, Gotoh S, Makihara N, Fukuhara M, Awano H, Isomura T, Suzuki K, Yasaka M, Okada Y, Kiyohara Y, Kitazono T. Matsuo R, et al. BMC Neurol. 2013 Apr 8;13:32. doi: 10.1186/1471-2377-13-32. BMC Neurol. 2013. PMID: 23566234 Free PMC article. - Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet.
Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Bernier M, et al. Aging (Albany NY). 2016 May;8(5):899-916. doi: 10.18632/aging.100942. Aging (Albany NY). 2016. PMID: 27070252 Free PMC article.
References
- Kawamata, T.S.E., and Finklestein, S.P. 1997. The role of polypeptide growth factors in recovery from stroke. In Brain plasticity. H.J. Freund, B.A. Sabel, and O.W. Witte, editors. Lippincott-Raven. Philadelphia, Pennsylvania, USA. 377–382. - PubMed
- Pons TP. Reorganizing the brain. Nat Med. 1998;4:561–562. - PubMed
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. - PubMed
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–189. - PubMed
- Cramer SC, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical