Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma. Evidence for its role in colonic tumorigenesis - PubMed (original) (raw)
Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma. Evidence for its role in colonic tumorigenesis
Y Friedmann et al. Am J Pathol. 2000 Oct.
Abstract
The human heparanase gene, an endo-beta-glucuronidase that cleaves heparan sulfate at specific intrachain sites, has recently been cloned and shown to function in tumor progression and metastatic spread. Antisense digoxigenin-labeled heparanase RNA probe and monoclonal anti-human heparanase antibodies were used to examine the expression of the heparanase gene and protein in normal, dysplastic, and neoplastic human colonic mucosa. To our knowledge, this is the first systematic study of heparanase expression in human colon cancer. Both the heparanase gene and protein were expressed at early stages of neoplasia, already at the stage of adenoma, but were practically not detected in the adjacent normal-looking colon epithelium. Gradually increasing expression of heparanase was evident as the cells progressed from severe dysplasia through well-differentiated to poorly differentiated colon carcinoma. Deeply invading colon carcinoma cells showed the highest levels of the heparanase mRNA and protein associated with expression of both the gene and enzyme by adjacent desmoplastic stromal fibroblasts. A high expression was also found in colon carcinoma metastases to lung, liver, and lymph nodes, as well as in the accompanying stromal fibroblasts. Moreover, extracts derived from tumor tissue expressed much higher levels of the heparanase protein and activity as compared to the normal colon tissue. In all specimens, the heparanase gene and protein exhibited the same pattern of expression. These results suggest a role of heparanase in colon cancer progression and may have both prognostic and therapeutic applications.
Figures
Figure 1.
Immunohistochemical analysis of the heparanase protein in neoplastic colon. Staining was performed as described in Materials and Methods. Positive staining is reddish-brown. Counterstain of nuclei is blue-purple. A: Heparanase immunostaining of tumor tissue (arrow) and lack of staining in the adjacent normal-looking tissue (arrowhead). A few macrophages in the normal-looking tissue (*) are positive. Cell surface expression of heparanase is demonstrated in the inset of the figure. B: Staining of blood vessels (arrows) and macrophages (arrowheads) in the proximity of the tumor. C and D: Sections of tubulovillous adenoma representing several presumed stages in tumor progression ranging from very mild dysplasia (arrows) in which almost no heparanase protein is detected, through moderate and severe dysplasia (arrowheads) showing a stronger stain, to a carcinomatous area (concaved arrows) in which high levels of heparanase are detected. E: Heparanase staining of well-differentiated (arrows) and more intense staining of poorly differentiated (arrowheads) adenocarcinoma. F: Highly positive heparanase staining of deeply invading tumor cells as well as of the desmoplastic stromal cells surrounding the tumor cells. G and H: Metastases of colon carcinoma derived from two different patients, in a regional lymph node (G) and in lung (H), showing high cytoplasmic expression of heparanase in the metastatic cells. Original magnifications: ×200 (A, C, and D); ×400 (B, E, F, and H); ×100 (G); ×1,000 (inset in A).
Figure 2.
Heparanase mRNA (in situ hybridization) in neoplastic colon tissue. Hybridization was performed as described in Materials and Methods. Positive labeling is brown-black. Nuclei counterstain is blue. A: High levels of heparanase mRNA in the superficially situated, severely dysplastic cells (arrows) of a tubulovillous adenoma relative to a faint labeling of mildly dysplastic cells (arrowheads). No heparanase mRNA is detected in the normal-looking epithelium (concaved arrows). B: A well-differentiated adenocarcinoma showing intense heparanase mRNA expression in the cytoplasm of the tumor cells. No heparanase mRNA is detected in the majority of the stromal fibroblasts. C: A poorly differentiated adenocarcinoma expressing heparanase mRNA both in the tumor cells and stromal fibroblasts. D: Heparanase mRNA expression in tumor tissue (arrows) and no expression in the adjacent normal-looking tissue (arrowheads). E: Deeply invading tumor cells and their surrounding stroma express heparanase mRNA. F: Metastatic colon cells in a regional lymph node showing high levels of heparanase mRNA. The surrounding stroma also expresses heparanase mRNA. Original magnifications: ×50 (A), ×200 (B), ×400 (C and F), and ×100 (D and E).
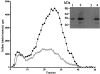
Figure 3.
Expression of heparanase activity and protein in colon carcinoma versus normal-looking colon tissue. Partially purified samples derived from normal-looking colon tissue (open diamonds; lanes 1 and 3) and from the colon tumor (filled diamonds; lanes 2 and 4) of the same patient were 1) tested for heparanase activity and 2) subjected to Western blot analysis using monoclonal anti-heparanase antibodies (inset). For heparanase activity, samples containing 15 μg of protein were incubated (24 hours at 37°C, pH 6.2) with sulfate-labeled ECM. The incubation medium was then subjected to gel filtration on Sepharose 6B. Similar results were obtained using tumor and normal tissues from three different patients. The amount of protein applied in lanes 1 and 2 was 0.9 μg/well versus 0.3 μg protein/well in lanes 3 and 4.
Similar articles
- Spatial and temporal heparanase expression in colon mucosa throughout the adenoma-carcinoma sequence.
Doviner V, Maly B, Kaplan V, Gingis-Velitski S, Ilan N, Vlodavsky I, Sherman Y. Doviner V, et al. Mod Pathol. 2006 Jun;19(6):878-88. doi: 10.1038/modpathol.3800603. Mod Pathol. 2006. PMID: 16607375 - Normal colon tissue and colon carcinoma show no difference in heparanase promoter methylation.
Peerless Y, Simon E, Sabo E, Ben-Izhak O, Hershkovitz D. Peerless Y, et al. Exp Mol Pathol. 2013 Apr;94(2):309-13. doi: 10.1016/j.yexmp.2013.01.001. Epub 2013 Jan 10. Exp Mol Pathol. 2013. PMID: 23313782 - Expression of serum amyloid A, in normal, dysplastic, and neoplastic human colonic mucosa: implication for a role in colonic tumorigenesis.
Gutfeld O, Prus D, Ackerman Z, Dishon S, Linke RP, Levin M, Urieli-Shoval S. Gutfeld O, et al. J Histochem Cytochem. 2006 Jan;54(1):63-73. doi: 10.1369/jhc.5A6645.2005. Epub 2005 Aug 22. J Histochem Cytochem. 2006. PMID: 16116035 - Heparanase enzyme in chronic inflammatory bowel disease and colon cancer.
Hermano E, Lerner I, Elkin M. Hermano E, et al. Cell Mol Life Sci. 2012 Aug;69(15):2501-13. doi: 10.1007/s00018-012-0930-8. Epub 2012 Feb 14. Cell Mol Life Sci. 2012. PMID: 22331282 Free PMC article. Review. - The role of heparanase in gastrointestinal cancer (Review).
Naomoto Y, Takaoka M, Okawa T, Nobuhisa T, Gunduz M, Tanaka N. Naomoto Y, et al. Oncol Rep. 2005 Jul;14(1):3-8. Oncol Rep. 2005. PMID: 15944760 Review.
Cited by
- Polymorphisms and a haplotype in heparanase gene associations with the progression and prognosis of gastric cancer in a northern Chinese population.
Li AL, Song YX, Wang ZN, Gao P, Miao Y, Zhu JL, Yue ZY, Xu HM. Li AL, et al. PLoS One. 2012;7(1):e30277. doi: 10.1371/journal.pone.0030277. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276173 Free PMC article. - Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas.
Mikami S, Ohashi K, Usui Y, Nemoto T, Katsube K, Yanagishita M, Nakajima M, Nakamura K, Koike M. Mikami S, et al. Jpn J Cancer Res. 2001 Oct;92(10):1062-73. doi: 10.1111/j.1349-7006.2001.tb01061.x. Jpn J Cancer Res. 2001. PMID: 11676857 Free PMC article. - Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis.
Barash U, Cohen-Kaplan V, Dowek I, Sanderson RD, Ilan N, Vlodavsky I. Barash U, et al. FEBS J. 2010 Oct;277(19):3890-903. doi: 10.1111/j.1742-4658.2010.07799.x. Epub 2010 Aug 31. FEBS J. 2010. PMID: 20840586 Free PMC article. Review. - Syndecan-1-Dependent Regulation of Heparanase Affects Invasiveness, Stem Cell Properties, and Therapeutic Resistance of Caco2 Colon Cancer Cells.
Katakam SK, Pelucchi P, Cocola C, Reinbold R, Vlodavsky I, Greve B, Götte M. Katakam SK, et al. Front Oncol. 2020 May 14;10:774. doi: 10.3389/fonc.2020.00774. eCollection 2020. Front Oncol. 2020. PMID: 32477959 Free PMC article. - Heparanase and coagulation-new insights.
Nadir Y. Nadir Y. Rambam Maimonides Med J. 2014 Oct 29;5(4):e0031. doi: 10.5041/RMMJ.10165. eCollection 2014 Oct. Rambam Maimonides Med J. 2014. PMID: 25386347 Free PMC article.
References
- Poste G, Fidler I: The pathogenesis of cancer metastases. Nature 1980, 283:139-146 - PubMed
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA: Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993, 9:541-573 - PubMed
- Duffy MJ: The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis 1992, 10:145-155 - PubMed
- Nakajima M, Irimura T, Nicolson GL: Heparanase and tumor metastasis. J Cell Biochem 1988, 36:157-167 - PubMed
- Vlodavsky I, Mohsen M, Lider O, Svahn CM, Ekre HP, Vigoda M, Ishai-Michaeli R, Peretz T: Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Invasion Metastasis 1995, 14:290-302 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical