Murine oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo - PubMed (original) (raw)
Murine oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo
C Langdon et al. Am J Pathol. 2000 Oct.
Abstract
Oncostatin M (OSM) is a multifunctional cytokine, a member of the interleukin-6/leukemia inhibitory factor (IL-6/LIF) family, that can regulate a number of connective-tissue cell types in vitro including cartilage and synovial tissue-derived fibroblasts, however its role in joint inflammation in vivo is not clear. We have analyzed murine OSM (muOSM) activity in vitro and in vivo in mouse joint tissue, to determine the potential role of this cytokine in local joint inflammation and pathology. The effects of muOSM and other IL-6/LIF cytokines on mouse synovial fibroblast cultures were assessed in vitro and showed induction of monocyte chemotactic protein-1, interleukin-6, and tissue inhibitor metalloproteinase-1, as well as enhancement of colony growth in soft agarose culture. Other IL-6/LIF cytokines including IL-6, LIF, or cardiotrophin-1, did not have such effects when tested at relatively high concentrations (20 ng/ml). To assess effects of muOSM in articular joints in vivo, we used recombinant adenovirus expressing muOSM cDNA (AdmuOSM) and injected purified recombinant virus (10(6) to 10(8) pfu) intra-articularly into the knees of various mouse strains. Histological analysis revealed dramatic alterations in the synovium but not in synovium of knees treated with the control virus Ad-dl70 or knees treated with Adm-IL-6 encoding biologically active murine IL-6. AdmuOSM effects were characterized by increases in the synovial cell proliferation, infiltration of mononuclear cells, and increases in extracellular matrix deposition that were evident at day 4, but much more marked at days 7, 14, and 21 after administration. The synovium took on characteristics similar to pannus and appeared to contact and invade cartilage. Collectively, these results provide good evidence that OSM regulates synovial fibroblast function differently than other IL-6-type cytokines, and can induce a proliferative invasive phenotype of synovium in vivo in mice on overexpression. We suggest that OSM may contribute to pathology in arthritis.
Figures
Figure 1.
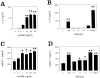
muOSM stimulates IL-6 and MCP-1 protein production by primary mouse synovial fibroblasts. Primary mouse synovial fibroblasts were cultured in 24-well dishes and stimulated with increasing concentrations of muOSM (0 to 50 ng/ml) (A and B). Supernatants were collected after 24 hours, stored at −20°C and then analyzed for IL-6 by the B9 hybridoma proliferation assay (A) and for mMCP-1 by ELISA (B). In addition, activity was compared to other IL-6/LIF cytokines and IL-1 in separate cell cultures (C and D), stimulated as indicated with muOSM (OSM), mIL-6 (IL-6), mLIF (LIF), or mCT-1 (CT-1) at 20 ng/ml and/or mIL-1β at 5 ng/ml. Supernatants were collected and analyzed as above. Data shows the mean and SD of triplicate treatments in one of two separate experiments that gave similar results. One star indicates significant difference from control (P < 0.05), whereas two stars indicate significance at P < 0.01, assessed by analysis of variance as in the Methods section. n.d., (not determined). IL-6 levels were not determined in supernatants from cells stimulated with IL-6.
Figure 2.
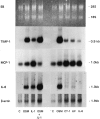
Regulation of mRNA in primary mouse synovial fibroblast cells. Primary mouse synovial fibroblast cells were stimulated for 22 hours with the indicated cytokines in two separate experiments (left and right panels). Total RNA was extracted and Northern blots were probed with mTIMP-1 and mMCP-1/JE oligonucleotides as well as a cDNA probe to rat IL-6. β-actin mRNA levels served as a control for RNA loading. Cytokine concentrations were 20 ng/ml muOSM (OSM), mCT-1 (CT-1), mLIF (LIF), and mIL-6 (IL-6) and 5 ng/ml mIL-1β (IL-1).
Figure 3.
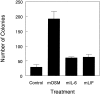
OSM stimulates anchorage-independent growth. Single cell suspensions of mouse synovial fibroblasts were suspended in 0.4% agarose in Dulbecco’s modified minimal essential medium containing 20% fetal bovine serum and the indicated cytokines at a concentration of 10 ng/ml. All treatments were completed in triplicate. After 14 to 17 days, the resultant cell colonies were optically graded for size as being 0 to 20 μm in diameter (single cells or small groups, white bars), of >20 μm (true colonies, black bars). Data shown is representative of two separate experiments.
Figure 4.
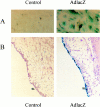
AdlacZ infection of synovial cells in vitro and in vivo. A: Primary mouse synovial fibroblasts were untreated or infected with AdmCMVlacZ or AdmuOSM at multiplicity of infection of 10 and 5. Fibroblasts were stained for lacZ expression with X-gal. The blue staining demonstrates expression of lacZ. B: Mice were treated with intra-articular AdlacZ (5 × 10 pfu) and sacrificed 24 hours later. Joints were removed, fixed, decalcified, and stained for lacZ expression. Blue staining was consistently observed in synovial lining cells.
Figure 5.
Histopathology of mouse knee joints. Adenovirus vectors were injected into the knee joints of mice, and the animals were sacrificed at various times following administration. Knee joints were fixed, decalcified, paraffin-embedded, and stained using H&E and EVG techniques. Tissues were assessed using pathological criteria as shown in Table 1 ▶ . Original magnification: ×100 (A–D and F); ×200 (E, G, and H). A: Contralateral joint of AdmuOSM, day 7. A, Adipocytes; M, meniscus; C, articular cartilage; T, tendon. Note the normal appearance and thickness of the synovial lining (arrow). B: Addl70, day 7. The synovial lining shows little if any thickening (arrow). C: AdmuOSM, day 4. Note the substantial increase in the thickness of the synovial lining (arrow). D: AdmuOSM, day 7. The synovial lining is extensively thickened and the entire synovium is infiltrated with mononuclear and some polymorphonuclear cells (PMN), with a concomitant decrease in adipocyte presence. Note the eroded miniscal outer surface (arrow). E: AdmuOSM, day 7. Higher magnification shows infiltration of the synovium, the PMN being clearly visible (open arrowheads). Note also the erosion of the articular cartilage adjacent to synovial tissue (closed arrowhead). F: AdmIL-6, day 7. The synovial lining shows a minor increase in thickness (arrow). G and H: AdmuOSM, day 7, showing matching fields stained with H&E and EVG, respectively. The synovial tissue has eroded the articular cartilage and underlying bone, and has penetrated the bone marrow (BM) (large arrowhead).
Similar articles
- Oncostatin M stimulates monocyte chemoattractant protein-1- and interleukin-1-induced matrix metalloproteinase-1 production by human synovial fibroblasts in vitro.
Langdon C, Leith J, Smith F, Richards CD. Langdon C, et al. Arthritis Rheum. 1997 Dec;40(12):2139-46. doi: 10.1002/art.1780401207. Arthritis Rheum. 1997. PMID: 9416850 - Oncostatin M regulates eotaxin expression in fibroblasts and eosinophilic inflammation in C57BL/6 mice.
Langdon C, Kerr C, Tong L, Richards CD. Langdon C, et al. J Immunol. 2003 Jan 1;170(1):548-55. doi: 10.4049/jimmunol.170.1.548. J Immunol. 2003. PMID: 12496442 - Adenovirus vector expressing mouse oncostatin M induces acute-phase proteins and TIMP-1 expression in vivo in mice.
Kerr C, Langdon C, Graham F, Gauldie J, Hara T, Richards CD. Kerr C, et al. J Interferon Cytokine Res. 1999 Oct;19(10):1195-205. doi: 10.1089/107999099313145. J Interferon Cytokine Res. 1999. PMID: 10547160 - Adenoviral gene transfer of interleukin-1 in combination with oncostatin M induces significant joint damage in a murine model.
Rowan AD, Hui W, Cawston TE, Richards CD. Rowan AD, et al. Am J Pathol. 2003 Jun;162(6):1975-84. doi: 10.1016/S0002-9440(10)64330-1. Am J Pathol. 2003. PMID: 12759253 Free PMC article. - Regulation of tissue inhibitor of metalloproteinase-1 in fibroblasts and acute phase proteins in hepatocytes in vitro by mouse oncostatin M, cardiotrophin-1, and IL-6.
Richards CD, Kerr C, Tanaka M, Hara T, Miyajima A, Pennica D, Botelho F, Langdon CM. Richards CD, et al. J Immunol. 1997 Sep 1;159(5):2431-7. J Immunol. 1997. PMID: 9278335
Cited by
- Oncostatin M-driven macrophage-fibroblast circuits as a drug target in autoimmune arthritis.
Huynh NC, Ling R, Komagamine M, Shi T, Tsukasaki M, Matsuda K, Okamoto K, Asano T, Muro R, Pluemsakunthai W, Kollias G, Kaneko Y, Takeuchi T, Tanaka S, Komatsu N, Takayanagi H. Huynh NC, et al. Inflamm Regen. 2024 Jul 31;44(1):36. doi: 10.1186/s41232-024-00347-0. Inflamm Regen. 2024. PMID: 39080781 Free PMC article. - IGF-1 Genome-Edited Human MSCs Exhibit Robust Anti-Arthritogenicity in Collagen-Induced Arthritis.
Chae DS, Han S, Kim SW. Chae DS, et al. Int J Mol Sci. 2024 Apr 18;25(8):4442. doi: 10.3390/ijms25084442. Int J Mol Sci. 2024. PMID: 38674027 Free PMC article. - Oncostatin M: Dual Regulator of the Skeletal and Hematopoietic Systems.
Sims NA, Lévesque JP. Sims NA, et al. Curr Osteoporos Rep. 2024 Feb;22(1):80-95. doi: 10.1007/s11914-023-00837-z. Epub 2024 Jan 10. Curr Osteoporos Rep. 2024. PMID: 38198032 Free PMC article. Review. - Multifaceted oncostatin M: novel roles and therapeutic potential of the oncostatin M signaling in rheumatoid arthritis.
Han L, Yan J, Li T, Lin W, Huang Y, Shen P, Ba X, Huang Y, Qin K, Geng Y, Wang H, Zheng K, Liu Y, Wang Y, Chen Z, Tu S. Han L, et al. Front Immunol. 2023 Nov 1;14:1258765. doi: 10.3389/fimmu.2023.1258765. eCollection 2023. Front Immunol. 2023. PMID: 38022540 Free PMC article. Review. - Role of Interleukin-6 Family Cytokines in Organ Fibrosis.
Chen Y, Zhou J, Xu S, Nie J. Chen Y, et al. Kidney Dis (Basel). 2023 Mar 22;9(4):239-253. doi: 10.1159/000530288. eCollection 2023 Aug. Kidney Dis (Basel). 2023. PMID: 37900004 Free PMC article. Review.
References
- Feldmann M, Brennan FM, Maini RN: Rheumatoid arthritis. Cell 1996, 85:307-310 - PubMed
- Miossec P, van den Berg W: Th1/Th2 cytokine balance in arthritis. Arthritis Rheum 1997, 40:2105-2115 - PubMed
- Arend WP, Dayer J: Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum 1995, 38:151-160 - PubMed
- Dinarello CA: Biological basis for interleukin-1 in disease. Blood 1996, 87:2095-2147 - PubMed
- Bruce G, Linsley P, Rose T: Oncostatin M. Prog Growth Factor Res 1992, 4:157-170 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials