Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9 - PubMed (original) (raw)
Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9
A K Lalwani et al. Am J Hum Genet. 2000 Nov.
Abstract
The authors had previously mapped a new locus-DFNA17, for nonsyndromic hereditary hearing impairment-to chromosome 22q12.2-q13. 3. DFNA17 spans a 17- to 23-cM region, and MYH9, a nonmuscle-myosin heavy-chain gene, is located within the linked region. Because of the importance of myosins in hearing, MYH9 was tested as a candidate gene for DFNA17. Expression of MYH9 in the rat cochlea was confirmed using reverse transcriptase-PCR and immunohistochemistry. MYH9 was immunolocalized in the organ of Corti, the subcentral region of the spiral ligament, and the Reissner membrane. Sequence analysis of MYH9 in a family with DFNA17 identified, at nucleotide 2114, a G-->A transposition that cosegregated with the inherited autosomal dominant hearing impairment. This missense mutation changes codon 705 from an invariant arginine (R) to histidine (H), R705H, within a highly conserved SH1 linker region. Previous studies have shown that modification of amino acid residues within the SH1 helix causes dysfunction of the ATPase activity of the motor domain in myosin II. Both the precise role of MYH9 in the cochlea and the mechanism by which the R705H mutation leads to the DFNA17 phenotype (progressive hearing impairment and cochleosaccular degeneration) remain to be elucidated.
Figures
Figure 1
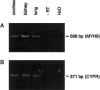
RT-PCR analysis of MYH9 expression in rat cochlear cDNA. cDNA from several tissues, including the cochlea, was assayed for MYH9 expression via PCR using MYH9-specific primers. A, cDNA from the cochlea, kidney, and lung. All samples yielded the expected 698-bp fragment amplified using rat MYH9-specific primers. Cochlear RNA, in the absence of RT, and water (H2O) served as negative controls. B, cDNA from all tissues tested for MYH9 expression. All samples are positive for expression of CYPR, a housekeeping gene, using _CYPR_-specific primers that yield the expected 371-bp fragment.
Figure 2
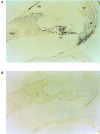
Immunolocalization of MYH9 in the rat cochlea. Radial sections of the rat cochlea were hybridized with anti-MYH9 antibody, were developed with a biotinylated, alkaline phosphatase–linked amplification system and were stained with NBT-BCIP. A, Photomicrograph of rat cochlear duct, illustrating the presence of anti-MYH9 immunoreactivity in several regions of the cochlea, including outer hair cells, cells of the organ of Corti (OC), the subcentral region of the spiral ligament (SL), and the Reissner membrane (RM). SGN = spiral ganglion neurons; SV = stria vascularis. B, The cochlear sections incubated without the anti-MYH9 antibody. The sections are free of immunoreactivity.
Figure 3
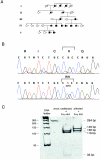
A, Pedigree with DFNA17. The affected members of five generations of the family with DFNA17 are denoted by blackened symbols. B, Sequence analysis of MYH9 in the family with DFNA17. MYH9 was sequenced in genomic DNA from the affected and unaffected members of the family with DFNA17. Although a single peak (G) is observed at nucleotide 2114 in the MYH9 sequence from the unaffected member V-2 (upper panel), a doublet (A/G) is observed at nucleotide 2114 (N) in the affected member III-4 (lower panel). The G→A transposition alters the codon from arginine (R) to histidine (H). C, Restriction analysis of MYH9 exon 16 2114G→A mutation eliminates the _Fnu_4HI restriction site, and this is reflected in the restriction digest pattern of MYH9 exon 16 from affected (III-4) and unaffected (V-2) individuals from the DFNA17 pedigree. The 284-bp PCR-amplified fragment contains three recognition sites for _Fnu_4HI—at 130, 135 and 168 bp from the 5′ end, of which the last site is absent in the mutant allele. Numbers at left are sizes of DNA ladder bands.
Figure 4
Alignment of the SH1 linker region in class II myosins. Alignment of peptide sequence spanning the SH1 linker region of muscle and nonmuscle class II myosins from seven species illustrates both the strict conservation of this linker region and the invariance of arginine (R705) in this sequence motif.
Similar articles
- Mutations in MYH9 exons 1, 16, 26, and 30 are infrequently found in Japanese patients with nonsyndromic deafness.
Kunishima S, Matsunaga T, Ito Y, Saito H. Kunishima S, et al. Genet Test Mol Biomarkers. 2009 Oct;13(5):705-7. doi: 10.1089/gtmb.2009.0044. Genet Test Mol Biomarkers. 2009. PMID: 19645626 - Absence of hearing loss in a mouse model for DFNA17 and MYH9-related disease: the use of public gene-targeted ES cell resources.
Parker LL, Gao J, Zuo J. Parker LL, et al. Brain Res. 2006 May 26;1091(1):235-42. doi: 10.1016/j.brainres.2006.03.032. Epub 2006 Apr 21. Brain Res. 2006. PMID: 16630581 - R705H mutation of MYH9 is associated with MYH9-related disease and not only with non-syndromic deafness DFNA17.
Verver E, Pecci A, De Rocco D, Ryhänen S, Barozzi S, Kunst H, Topsakal V, Savoia A. Verver E, et al. Clin Genet. 2015 Jul;88(1):85-9. doi: 10.1111/cge.12438. Epub 2014 Jul 26. Clin Genet. 2015. PMID: 24890873 - Advances in the understanding of MYH9 disorders.
Kunishima S, Saito H. Kunishima S, et al. Curr Opin Hematol. 2010 Sep;17(5):405-10. doi: 10.1097/MOH.0b013e32833c069c. Curr Opin Hematol. 2010. PMID: 20601875 Review. - Unconventional myosins and the genetics of hearing loss.
Friedman TB, Sellers JR, Avraham KB. Friedman TB, et al. Am J Med Genet. 1999 Sep 24;89(3):147-57. doi: 10.1002/(sici)1096-8628(19990924)89:3<147::aid-ajmg5>3.0.co;2-6. Am J Med Genet. 1999. PMID: 10704189 Review.
Cited by
- Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear.
Bird JE, Daudet N, Warchol ME, Gale JE. Bird JE, et al. J Neurosci. 2010 Sep 15;30(37):12545-56. doi: 10.1523/JNEUROSCI.3042-10.2010. J Neurosci. 2010. PMID: 20844149 Free PMC article. - Mutation screening in non-syndromic hearing loss patients with cochlear implantation by massive parallel sequencing in Taiwan.
Liu WH, Chang PY, Chang SC, Lu JJ, Wu CM. Liu WH, et al. PLoS One. 2019 Jan 25;14(1):e0211261. doi: 10.1371/journal.pone.0211261. eCollection 2019. PLoS One. 2019. PMID: 30682115 Free PMC article. - Identification of Novel and Recurrent Variants in MYO15A in Ashkenazi Jewish Patients With Autosomal Recessive Nonsyndromic Hearing Loss.
Booth KT, Hirsch Y, Vardaro AC, Ekstein J, Yefet D, Quint A, Weiden T, Corey DP. Booth KT, et al. Front Genet. 2021 Oct 18;12:737782. doi: 10.3389/fgene.2021.737782. eCollection 2021. Front Genet. 2021. PMID: 34733312 Free PMC article. - Function and expression pattern of nonsyndromic deafness genes.
Hilgert N, Smith RJ, Van Camp G. Hilgert N, et al. Curr Mol Med. 2009 Jun;9(5):546-64. doi: 10.2174/156652409788488775. Curr Mol Med. 2009. PMID: 19601806 Free PMC article. Review. - MYH9: Structure, functions and role of non-muscle myosin IIA in human disease.
Pecci A, Ma X, Savoia A, Adelstein RS. Pecci A, et al. Gene. 2018 Jul 20;664:152-167. doi: 10.1016/j.gene.2018.04.048. Epub 2018 Apr 19. Gene. 2018. PMID: 29679756 Free PMC article. Review.
References
Electronic-Database Information
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank (for rat myh9 sequence [accession number GI 6981235])
- Genome Database, http://www.gdb.org
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/omim (for DFNA17 [MIM 603622])
- Primer3 software, http://www.genome.wi.mit.edu
References
- Avraham KB, Hasson T, Sobe T, Balsara B, Testa JR, Skvorak AB, Morton CC, Copeland NG, Jenkins NA (1997) Characterization of unconventional MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice. Hum Mol Genet 6:1225–1231 - PubMed
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA (1995) The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet 11:369–375 - PubMed
- Collins FS (1995) Positional cloning moves from perditional to traditional. Nat Genet 9:347–350 - PubMed
- De Lozanne A, Spudich JA (1987) Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236:1086–1091 - PubMed
- Dunham I, Shimizu N, Roe BA, Chissoe S, Hunt AR, Collins JE, Bruskiewich R, et al (1999) The DNA sequence of human chromosome 22. Nature 402:489–495 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous