Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function - PubMed (original) (raw)
Review
Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function
A O Tzianabos. Clin Microbiol Rev. 2000 Oct.
Abstract
Polysaccharide immunomodulators were first discovered over 40 years ago. Although very few have been rigorously studied, recent reports have revealed the mechanism of action and structure-function attributes of some of these molecules. Certain polysaccharide immunomodulators have been identified that have profound effects in the regulation of immune responses during the progression of infectious diseases, and studies have begun to define structural aspects of these molecules that govern their function and interaction with cells of the host immune system. These polymers can influence innate and cell-mediated immunity through interactions with T cells, monocytes, macrophages, and polymorphonuclear lymphocytes. The ability to modulate the immune response in an appropriate way can enhance the host's immune response to certain infections. In addition, this strategy can be utilized to augment current treatment regimens such as antimicrobial therapy that are becoming less efficacious with the advent of antibiotic resistance. This review focuses on recent studies that illustrate the structural and biologic activities of specific polysaccharide immunomodulators and outlines their potential for clinical use.
Figures
FIG. 1
Schematic representation of the balance between pro- and anti-inflammatory cytokines elicited during bacterial sepsis. The onset of bacterial sepsis immediately leads to the production of numerous proinflammatory mediators, such as TNF-α, IL-1β, IL-6, IL-8, and NO. To prevent an overwhelming inflammatory response anti-inflammatory mediators such as IL-10 and MCP-1 are produced a few hours later. These cytokines have pleiotropic effects functioning to directly inhibit proinflammatory cytokine synthesis and promote the synthesis of specific cytokine inhibitors, such as IL-1 receptor antagonist and soluble TNF receptors. In addition, they downregulate chemokine and chemokine receptor production and inhibit Th1 cytokines, such as IL-2 and IFN-γ.
FIG. 2
Structure of B. fragilis PS A. This repeating unit comprises a trisaccharide backbone with a galactofuranose side chain. PS A has a positively charged free amino group on the 2,4-dideoxy-4-amino-
d
-FucNAc and a 4,6-pyruvate ring on the galactose moiety.
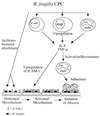
FIG. 3
Proposed model of intra-abdominal abscess formation. The CPC of B. fragilis interacts with cells of the host immune system within the peritoneal cavity. This interaction (i) allows for the localization of B. fragilis within the abdominal cavity, thus resulting in enhanced adherence to the mesothelial surface and the ability to resist clearance from the peritoneum, and (ii) stimulates proinflammatory cytokines and chemokines, the production of which stimulates the expression of CAMs (such as ICAM-1) on host cells and the recruitment of PMNs to the abdominal cavity. Infiltration and sequestration of PMNs within the peritoneal cavity are the hallmark of intra-abdominal abscess formation. The CPC of B. fragilis interacts with T cells, peritoneal macrophages, and PMNs. In response to this interaction, these cells produce TNF-α and IL-8, which serve to recruit activated PMNs to the peritoneal cavity and upregulate ICAM-1 expression on mesothelial cells. The production of ICAM-1 by mesothelial cells serves as a functional ligand for infiltrating PMNs. The recruitment of PMNs into the peritoneal cavity and subsequent adherence of these cells to activated mesothelial tissue form the first stages of intra-abdominal abscess formation in the infected host.
FIG. 4
Polysaccharide-mediated protection against mortality and abscess formation associated with experimental intra-abdominal sepsis (62). Animals were treated prophylactically with saline, PGG-glucan, PS A, or a combination of these polymers and challenged with rat cecal contents. The challenge inoculum is titrated to yield approximately a 50% mortality rate with 100% abscess formation in surviving animals. Results are taken from two separate experiments. (A) Mortality rates in animals treated with PGG-glucan or with the combination of PGG-glucan and PS A were significantly reduced (∗) compared with the saline-treated control group. (B) Abscess formation was significantly reduced (∗) in animals treated with PS A or the combination of PS A and PGG-glucan compared with the saline-treated controls. These results demonstrate that administration of two polysaccharide immunomodulators can prevent both phases of intra-abdominal sepsis in the absence of antibiotic therapy.
Similar articles
- Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities.
Meng X, Liang H, Luo L. Meng X, et al. Carbohydr Res. 2016 Apr 7;424:30-41. doi: 10.1016/j.carres.2016.02.008. Epub 2016 Mar 2. Carbohydr Res. 2016. PMID: 26974354 Review. - Botanical polysaccharides: macrophage immunomodulation and therapeutic potential.
Schepetkin IA, Quinn MT. Schepetkin IA, et al. Int Immunopharmacol. 2006 Mar;6(3):317-33. doi: 10.1016/j.intimp.2005.10.005. Epub 2005 Nov 10. Int Immunopharmacol. 2006. PMID: 16428067 Review. - Polysaccharides as vaccine adjuvants.
Sun B, Yu S, Zhao D, Guo S, Wang X, Zhao K. Sun B, et al. Vaccine. 2018 Aug 23;36(35):5226-5234. doi: 10.1016/j.vaccine.2018.07.040. Epub 2018 Jul 26. Vaccine. 2018. PMID: 30057282 Review. - Immunomodulators as adjuvants for vaccines and antimicrobial therapy.
Nicholls EF, Madera L, Hancock RE. Nicholls EF, et al. Ann N Y Acad Sci. 2010 Dec;1213:46-61. doi: 10.1111/j.1749-6632.2010.05787.x. Epub 2010 Oct 4. Ann N Y Acad Sci. 2010. PMID: 20946578 Review. - Application of chemical immunomodulators to the treatment of cancer and AIDS.
Turowski RC, Triozzi PL. Turowski RC, et al. Cancer Invest. 1994;12(6):620-43. doi: 10.3109/07357909409023048. Cancer Invest. 1994. PMID: 7994598 Review.
Cited by
- An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides.
Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, Sun T, Zhang X, Zhao RJ, Gu L, Cao C, Zhou SF. Cheng J, et al. Drug Des Devel Ther. 2014 Dec 17;9:33-78. doi: 10.2147/DDDT.S72892. eCollection 2015. Drug Des Devel Ther. 2014. PMID: 25552899 Free PMC article. Review. - IM-133N - A Useful Herbal Combination for Eradicating Disease-triggering Pathogens in Mice via Immunotherapeutic Mechanisms.
Firashathulla S, Inamdar MN, Rafiq M, Viswanatha GL, Sharath Kumar LM, Babu UV, Ramakrishnan S, Paramesh R. Firashathulla S, et al. J Pharmacopuncture. 2016 Mar;19(1):21-7. doi: 10.3831/KPI.2016.19.003. J Pharmacopuncture. 2016. PMID: 27280046 Free PMC article. - Comparative nutritional characteristics of the three major Chinese Dendrobium species with different growth years.
Yuan Y, Yu M, Zhang B, Liu X, Zhang J. Yuan Y, et al. PLoS One. 2019 Sep 20;14(9):e0222666. doi: 10.1371/journal.pone.0222666. eCollection 2019. PLoS One. 2019. PMID: 31539401 Free PMC article. - Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties.
Jurášková D, Ribeiro SC, Silva CCG. Jurášková D, et al. Foods. 2022 Jan 8;11(2):156. doi: 10.3390/foods11020156. Foods. 2022. PMID: 35053888 Free PMC article. Review. - Structural Elucidation and Immunostimulatory Activities of Quinoa Non-starch Polysaccharide Before and After Deproteinization.
Cao RA, Ma N, Palanisamy S, Talapphet N, Zhang J, Wang C, You S. Cao RA, et al. J Polym Environ. 2022;30(6):2291-2303. doi: 10.1007/s10924-021-02335-8. Epub 2021 Nov 26. J Polym Environ. 2022. PMID: 34849108 Free PMC article.
References
- Adams D S, Pero S C, Petro J B, Nathans R, Mackin W M, Wakshull E. PGG-glucan activates NF-kappaB-like and NF-IL-6-like transcription factor complexes in a murine monocytic cell line. J Leukoc Biol. 1997;62:865–873. - PubMed
- Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
- Assenmacher M, Lohning M, Scheffold A, Manz R A, Schmitz J, Radbruch A. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur J Immunol. 1998;28:1534–1543. - PubMed
- Babineau T J, Hackford A, Kenler A, Bistrian B, Forse R A, Fairchild P G, Heard S, Keroack M, Caushaj P, Benotti P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch Surg. 1994;129:1204–1210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources