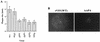Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release - PubMed (original) (raw)
Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release
H R Jayakar et al. J Virol. 2000 Nov.
Abstract
The N terminus of the matrix (M) protein of vesicular stomatitis virus (VSV) and of other rhabdoviruses contains a highly conserved PPPY sequence (or PY motif) similar to the late (L) domains in the Gag proteins of some retroviruses. These L domains in retroviral Gag proteins are required for efficient release of virus particles. In this report, we show that mutations in the PPPY sequence of the VSV M protein reduce virus yield by blocking a late stage in virus budding. We also observed a delay in the ability of mutant viruses to cause inhibition of host gene expression compared to wild-type (WT) VSV. The effect of PY mutations on virus budding appears to be due to a block at a stage just prior to virion release, since electron microscopic examination of PPPA mutant-infected cells showed a large number of assembled virions at the plasma membrane trapped in the process of budding. Deletion of the glycoprotein (G) in addition to these mutations further reduced the virus yield to less than 1% of WT levels, and very few particles were assembled at the cell surface. This observation suggested that G protein aids in the initial stage of budding, presumably during the formation of the bud site. Overall, our results confirm that the PPPY sequence of the VSV M protein possesses L domain activity analogous to that of the retroviral Gag proteins.
Figures
FIG. 1
Schematic representation of PPXY mutations in the MGF minigenome. MGF stands for the M, G, and GFP minigenome. l and t denote the leader and trailer sequences, respectively. The hepatitis delta virus ribozyme sequence (HDV) and the T7 terminator sequence (ΦT) are represented by hatched boxes. Arrows indicate the direction of transcription for each gene. The solid box at the 5′ end of the M gene represents the N-terminal region of the matrix protein including the PPXY motif, which is enlarged below. The numbering shows the positions of amino acids with respect to the N terminus of M protein. Alanine substitutions were made for the proline and tyrosine residues either singly or in combinations. The name of each mutant is listed on the right. WT stands for the wild-type sequence of the PY motif in VSV M protein. The mutants were classified into two groups, P and Y mutants, based on the severity of their phenotypes.
FIG. 2
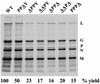
Assembly and budding profile of PPXY mutants. BHK-21 cells were infected with either the WT or mutant viruses at an MOI of 10. Cells were labeled at 7 h p.i. with [35S]methionine. At 15 h p.i., viruses were harvested from the supernatants by centrifugation and analyzed by SDS-PAGE followed by autoradiography. The positions of the five VSV proteins are indicated on the right. The mutated residue(s) in the PPPY sequence of each mutant is underlined. The percent yield of virus in this experiment is calculated based on the quantitation of N protein using ImageQuant software (Molecular Dynamics).
FIG. 3
Comparison of the plaque sizes of WT and PPXY mutants in BHK-21 cells. (A) A standard plaque assay was performed for each virus on BHK-21 cells. At 13 h p.i., the sizes of 25 individual plaques were determined for each virus. The mean plaque size for each virus is shown. The residue(s) changed in each mutant is underlined. (B) Fluorescence micrograph of GFP-expressing cells comparing the plaque sizes of WT and the PPPA mutant (magnification, ×7.5).
FIG. 4
Growth kinetics of PPXY mutants in BHK-21 cells. BHK-21 cells were infected with either the WT or the mutant viruses at an MOI of 10. At various times p.i., aliquots of supernatant were taken to determine the titer by a plaque assay on BHK cells. The kinetics for the P and Y mutants were determined simultaneously under similar conditions, but they were plotted individually to show the growth difference during early times of infection.
FIG. 5
Electron micrographs showing morphologies of PPXY mutants. BHK-21 cells infected with WT or mutant viruses were fixed at 8 h p.i., and thin sections of cells were examined under the electron microscope to determine the stage(s) at which morphogenesis of PPXY mutants was blocked. (A) WT; (B) ΔG-GFP; (C) AAPY; (D and E) PPPA; (F) ΔG-AAPA. Magnifications: ×15,000 for panels A through D; ×18,000 for panel F. Panel E) is an enlargement of panel D. Arrows in panel E point to the budding virions attached to the plasma membrane of the infected cell.
FIG. 6
Scanning electron micrographs of WT virus and the PPPA mutant. BHK-21 cells on plastic coverslips were infected with either WT virus or the PPPA mutant at an MOI of 10. At 8 h p.i., cells were fixed and processed as described in Materials and Methods. (A and D) Uninfected cells; (B and E) WT infected cells; (C and F) PPPA mutant-infected cells. Magnifications: ×4,000 for panels A through C; ×12,000 for panels D through F.
FIG. 7
Assembly phenotype of AAPA mutant in the absence of glycoprotein. Virions released from cells infected with either ΔG-GFP or ΔG-PPPA were compared to those released from WT-infected cells. Virions were prepared and analyzed on SDS-polyacrylamide gels as described for Fig. 2. (A) WT; (B) ΔG-GFP; (C) ΔG-PPPA; (D and E) longer exposures of panels B and C.
FIG. 8
Kinetics of host protein synthesis shutoff by the AAPA mutant. Cells were infected with either WT virus or the AAPA mutant (MUT) virus at an MOI of 10. At the times indicated, the cells were pulse-labeled for 15 min with [35S]methionine and lysed in detergent buffer. A portion of the cell lysate was analyzed by SDS-PAGE followed by autoradiography. Labeled uninfected cells (MOCK) served as a control. (B) The amount of 35S-labeled host proteins in each lane was determined by quantitating a representative area on the gel (indicated by the asterisk in panel A) with a STORM Phosphorimager and ImageQuant software (Molecular Dynamics). The amount of host proteins in the uninfected cells (control) was considered to be 100%. The extent of host shutoff was expressed as a percentage of the amount of host proteins in the control. The extent of host shutoff caused by the AAPA mutant at an MOI of 50 is shown in the inset (C).
Similar articles
- Budding of PPxY-containing rhabdoviruses is not dependent on host proteins TGS101 and VPS4A.
Irie T, Licata JM, McGettigan JP, Schnell MJ, Harty RN. Irie T, et al. J Virol. 2004 Mar;78(6):2657-65. doi: 10.1128/jvi.78.6.2657-2665.2004. J Virol. 2004. PMID: 14990685 Free PMC article. - Functional analysis of late-budding domain activity associated with the PSAP motif within the vesicular stomatitis virus M protein.
Irie T, Licata JM, Jayakar HR, Whitt MA, Bell P, Harty RN. Irie T, et al. J Virol. 2004 Jul;78(14):7823-7. doi: 10.1128/JVI.78.14.7823-7827.2004. J Virol. 2004. PMID: 15220457 Free PMC article. - A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding.
Harty RN, Paragas J, Sudol M, Palese P. Harty RN, et al. J Virol. 1999 Apr;73(4):2921-9. doi: 10.1128/JVI.73.4.2921-2929.1999. J Virol. 1999. PMID: 10074141 Free PMC article. - Late domain function identified in the vesicular stomatitis virus M protein by use of rhabdovirus-retrovirus chimeras.
Craven RC, Harty RN, Paragas J, Palese P, Wills JW. Craven RC, et al. J Virol. 1999 Apr;73(4):3359-65. doi: 10.1128/JVI.73.4.3359-3365.1999. J Virol. 1999. PMID: 10074190 Free PMC article. - [Envelope virus assembly and budding].
Irie T. Irie T. Uirusu. 2010 Jun;60(1):105-13. doi: 10.2222/jsv.60.105. Uirusu. 2010. PMID: 20848870 Review. Japanese.
Cited by
- Arenavirus budding resulting from viral-protein-associated cell membrane curvature.
Schley D, Whittaker RJ, Neuman BW. Schley D, et al. J R Soc Interface. 2013 Jul 17;10(86):20130403. doi: 10.1098/rsif.2013.0403. Print 2013 Sep 6. J R Soc Interface. 2013. PMID: 23864502 Free PMC article. - Identification of two additional translation products from the matrix (M) gene that contribute to vesicular stomatitis virus cytopathology.
Jayakar HR, Whitt MA. Jayakar HR, et al. J Virol. 2002 Aug;76(16):8011-8. doi: 10.1128/jvi.76.16.8011-8018.2002. J Virol. 2002. PMID: 12134006 Free PMC article. - A role for the C terminus of Mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles.
Shtanko O, Imai M, Goto H, Lukashevich IS, Neumann G, Watanabe T, Kawaoka Y. Shtanko O, et al. J Virol. 2010 May;84(10):5415-22. doi: 10.1128/JVI.02417-09. Epub 2010 Mar 3. J Virol. 2010. PMID: 20200234 Free PMC article. - Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins.
Martin-Serrano J, Yarovoy A, Perez-Caballero D, Bieniasz PD. Martin-Serrano J, et al. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12414-9. doi: 10.1073/pnas.2133846100. Epub 2003 Sep 30. Proc Natl Acad Sci U S A. 2003. PMID: 14519844 Free PMC article. - In vivo interference of Rous sarcoma virus budding by cis expression of a WW domain.
Patnaik A, Wills JW. Patnaik A, et al. J Virol. 2002 Mar;76(6):2789-95. doi: 10.1128/jvi.76.6.2789-2795.2002. J Virol. 2002. PMID: 11861846 Free PMC article.
References
- Ahmed M, Lyles D S. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology. 1997;237:378–388. - PubMed
- Andre B, Springael J Y. WWP, a new amino acid motif present in single or multiple copies in various proteins including dystrophin and the SH3-binding Yes-associated protein YAP65. Biochem Biophys Res Commun. 1994;205:1201–1205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources