Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells - PubMed (original) (raw)
Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells
P D Bieniasz et al. J Virol. 2000 Nov.
Abstract
The recent identification of human gene products that are required for early steps in the human immunodeficiency virus type 1 (HIV-1) life cycle has raised the possibility that rodents might be engineered to support HIV-1 infection. Therefore, we have examined the ability of modified mouse, rat, and hamster cell lines to support productive HIV-1 replication. Rodent cells, engineered to support Tat function by stable expression of a permissive cyclin T1 protein, proved to be able to support reverse transcription, integration, and early gene expression at levels comparable to those observed in human cell lines. Surprisingly, however, levels of CD4- and coreceptor-dependent virus entry were reduced to a variable but significant extent in both mouse and rat fibroblast cell lines. Additional posttranscriptional defects were observed, including a reduced level of unspliced HIV-1 genomic RNA and reduced structural gene expression. Furthermore, the HIV-1 Gag precursor is generally inefficiently processed and is poorly secreted from mouse and rat cells in a largely noninfectious form. These posttranscriptional defects, together, resulted in a dramatically reduced yield of infectious virus (up to 10,000-fold) over a single cycle of HIV-1 replication, as compared to human cells. Interestingly, these defects were less pronounced in one hamster cell line, CHO, which not only was able to produce infectious HIV-1 particles at a level close to that observed in human cells, but also could support transient, low-level HIV-1 replication. Importantly, the blocks to infectious virus production in mouse and rat cells are recessive, since they can be substantially suppressed by fusion with uninfected human cells. These studies imply the existence of one or more human gene products, either lacking or nonfunctional in most rodent cells that are critical for infectious HIV-1 virion morphogenesis.
Figures
FIG. 1
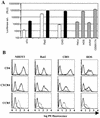
Rodent cell lines that express human gene products required for HIV-1 replication. (A) NIH 3T3, Rat2, and CHO cells either unmodified (open bars) or transduced with retroviral vectors that express mCycT1(Y261C) (solid bars). These cell lines, as well as various human cell lines (hatched bars) were infected with NL4-3E−luc, pseudotyped with the VSV-G envelope glycoprotein. Forty-eight hours after infection, luciferase activity in cell lysates was determined. RLU, relative light units. (B) The cell lines in panel A were sequentially transduced first with an LXSH-derived retrovirus vector that expresses CD4 and subsequently with pBABE Puro-derived vectors that express either CXCR4 or CCR5. The expression level of each of these proteins was determined by using PE-conjugated antibodies. CD4+ CXCR4+ cell lines are displayed with dark grey lines, and CD4+ CCR5+ cell lines are displayed with pale grey lines. The parental, mCycT1(Y261C)-expressing cell lines were used as negative controls (black lines). For comparison, HOS cells and the CD4- and coreceptor-positive derivatives (GHOST cell lines) were simultaneously analyzed.
FIG. 2
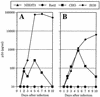
HIV-1 replication in mCycT1(Y261C)- and CD4-, and coreceptor-expressing rodent cell lines. (A) The CD4+ CXCR4+ NIH 3T3, Rat2, CHO, and HOS cell lines described in the legend to Fig. 1 were infected with NL/IIIB. Alternatively (B) CD4+ CCR5+ counterparts were infected with NL/JRFL. Viral replication was observed for the subsequent 10 days by monitoring the p24 concentration in the culture supernatants by ELISA.
FIG. 3
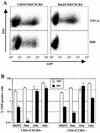
A target cell-specific, CD4- and coreceptor-dependent HIV-1 entry defect. (A) FACS analysis of GFP reporter virus infection of CD4+ CXCR4+ CHO and CD4+ CXCR4+ Rat2 cells, mediated by the VSV-G envelope (upper panels) or by the X4 tropic HIV-1IIIB envelope (lower panels). (B) Analyses identical to that shown in panel A were done with all of the CD4- and coreceptor-positive cell lines, using HIV-1IIIB enveloped GFP reporter virus for CD4+ CXCR4+ cells and a YU2 enveloped virus for CD4+ CCR5+ cell lines. The proportion of GFP-positive cells was determined by setting a gate at a fluorescent intensity of between 10 and 30, depending on the cell line, so that less than 5 positive events were observed in 50,000 mock-infected cells (0.01%).
FIG. 4
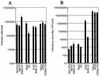
Posttranscriptional defects in infectious virus production from rodent cells during a single cycle of HIV-1 replication. (A) Rodent and human cells are similarly susceptible to HIV-1(VSV-G) infection. The indicated cell lines were infected with R7/E−/GFP pseudotyped with VSV-G. The number of infected cells per well was determined by multiplying the percentage of GFP-positive cells by the total number of cells in each well. (B) Cell lines were infected with replication-competent, X4-tropic but VSV-G-pseudotyped HIV-1IIIB. After extensive washing, infectious virus production was analyzed by using CD4+ CXCR4+ CHO cells and a focal immunoassay, as described in Materials and Methods. FFU, focus-forming units.
FIG. 5
Analysis of HIV-1 RNA in infected cells. The indicated HIV receptor-negative cell lines were infected with VSV-G-pseudotyped HIV-1 as described in the legend to Fig. 4B. Forty-eight hours later, total RNA was extracted from infected cells and analyzed by ribonuclease protection assay. Arrows indicate the predicted migration of the 317-nucleotide undigested probe (P) which spans the major 5′ splice donor site, resulting in two protected fragments of 262 and 213 nucleotides that correspond to unspliced (U) or spliced (S) HIV-1 RNA, respectively. The numbers below the lanes indicate the levels of each of the RNA species, determined by PhosphorImager analysis and expressed in arbitrary units. M, molecular size markers.
FIG. 6
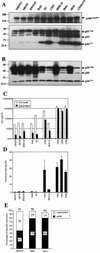
Reduced levels of Gag expression, processing, and assembly into infectious particles in rodent cell lines. The same cells that generated the infectious virus measured in Fig. 4B as well as the corresponding supernatant samples were analyzed for viral Gag protein content. (A) Equivalent quantities (10 μg) of cell lysate were separated on acrylamide gels and subjected to Western blot analysis with a p24-specific monoclonal antibody. Note that the upper panel, which displays the p160Gag-Pol precursor, represents a longer exposure than the lower panel. (B) Aliquots of cell lysates containing equivalent amounts of p24 (2.5 ng) as measured by p24 ELISA were analyzed by Western blotting as in panel A. (C) Viral p24 in cell lysates and supernatants as measured with a commercial ELISA kit. (D) The quantity of infectious virus produced relative to the total amount of supernatant p24 was calculated for each cell line. (E) Relative proportions of the total supernatant p24 that could be pelleted by ultracentrifugation through a sucrose cushion. Numbers refer to the percentage of the starting material that was recovered in each fraction and their sum.
FIG. 7
Fusion of HIV-1-infected rodent cells with uninfected human cells enhances infectious particle release. MDTF (A) or Rat2 (B) cells were infected with VSV-G-pseudotyped HIV-1 as described in the legend to Fig. 4. The following day, the cells were washed, trypsinized, and replated, either alone (None) or along with an equal number of MDTF, Rat2, or HOS cells. Cocultivated cells were fused with PEG, except where indicated, and subsequent infectious virus production was analyzed after 16 h.
Similar articles
- A block to human immunodeficiency virus type 1 assembly in murine cells.
Mariani R, Rutter G, Harris ME, Hope TJ, Kräusslich HG, Landau NR. Mariani R, et al. J Virol. 2000 Apr;74(8):3859-70. doi: 10.1128/jvi.74.8.3859-3870.2000. J Virol. 2000. PMID: 10729160 Free PMC article. - Ability of small animal cells to support the postintegration phase of human immunodeficiency virus type-1 replication.
Koito A, Shigekane H, Matsushita S. Koito A, et al. Virology. 2003 Jan 5;305(1):181-91. doi: 10.1006/viro.2002.1755. Virology. 2003. PMID: 12504551 - Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly.
Mariani R, Rasala BA, Rutter G, Wiegers K, Brandt SM, Kräusslich HG, Landau NR. Mariani R, et al. J Virol. 2001 Apr;75(7):3141-51. doi: 10.1128/JVI.75.7.3141-3151.2001. J Virol. 2001. PMID: 11238841 Free PMC article. - HIV-1 gag proteins: diverse functions in the virus life cycle.
Freed EO. Freed EO. Virology. 1998 Nov 10;251(1):1-15. doi: 10.1006/viro.1998.9398. Virology. 1998. PMID: 9813197 Review. - Cyclophilin and gag in HIV-1 replication and pathogenesis.
Franke EK, Luban J. Franke EK, et al. Adv Exp Med Biol. 1995;374:217-28. doi: 10.1007/978-1-4615-1995-9_19. Adv Exp Med Biol. 1995. PMID: 7572395 Review. No abstract available.
Cited by
- Mouse T-cells restrict replication of human immunodeficiency virus at the level of integration.
Tervo HM, Goffinet C, Keppler OT. Tervo HM, et al. Retrovirology. 2008 Jul 8;5:58. doi: 10.1186/1742-4690-5-58. Retrovirology. 2008. PMID: 18611257 Free PMC article. - Integrated Human Immunodeficiency Virus Type 1 Sequence in J-Lat 10.6.
Chung CH, Mele AR, Allen AG, Costello R, Dampier W, Nonnemacher MR, Wigdahl B. Chung CH, et al. Microbiol Resour Announc. 2020 Apr 30;9(18):e00179-20. doi: 10.1128/MRA.00179-20. Microbiol Resour Announc. 2020. PMID: 32354973 Free PMC article. - A participant-derived xenograft model of HIV enables long-term evaluation of autologous immunotherapies.
McCann CD, van Dorp CH, Danesh A, Ward AR, Dilling TR, Mota TM, Zale E, Stevenson EM, Patel S, Brumme CJ, Dong W, Jones DS, Andresen TL, Walker BD, Brumme ZL, Bollard CM, Perelson AS, Irvine DJ, Jones RB. McCann CD, et al. J Exp Med. 2021 Jul 5;218(7):e20201908. doi: 10.1084/jem.20201908. Epub 2021 May 14. J Exp Med. 2021. PMID: 33988715 Free PMC article. - Productive infection of primary murine astrocytes, lymphocytes, and macrophages by human immunodeficiency virus type 1 in culture.
Nitkiewicz J, Chao W, Bentsman G, Li J, Kim SY, Choi SY, Grunig G, Gelbard H, Potash MJ, Volsky DJ. Nitkiewicz J, et al. J Neurovirol. 2004 Dec;10(6):400-8. doi: 10.1080/13550280490890097. J Neurovirol. 2004. PMID: 15765811
References
- Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials