ICP0 induces the accumulation of colocalizing conjugated ubiquitin - PubMed (original) (raw)
ICP0 induces the accumulation of colocalizing conjugated ubiquitin
R D Everett. J Virol. 2000 Nov.
Abstract
Herpes simplex virus type 1 (HSV-1) immediate-early protein ICP0 is a general activator of viral gene expression which stimulates the initiation of lytic infection and reactivation from quiescence and latency. The importance of ICP0 to the biology of HSV-1 infection has stimulated interest in its mode of action. Previous studies have reported its interactions with other viral regulatory molecules, with the translation apparatus, with cyclin D3, and with a ubiquitin-specific protease. It has been demonstrated that ICP0 is able to induce the proteasome-dependent degradation of a number of cellular proteins, including components of centromeres and small nuclear substructures known as ND10 or PML nuclear bodies. ICP0 has a RING finger zinc-binding domain which is essential for its functions. In view of several recent examples of other RING finger proteins which modulate the stability of specific target proteins by acting as components of E3 ubiquitin ligase complexes, this study has explored whether ICP0 might operate via a similar mechanism. Evidence that the foci of accumulated ICP0 in transfected and infected cells contain enhanced levels of conjugated ubiquitin is presented. This effect was dependent on the RING finger region of ICP0, and comparison of the properties of a number of ICP0 mutants revealed an excellent correlation between previously established functions of ICP0 and its ability to induce concentrations of colocalizing conjugated ubiquitin. These results strongly support the hypothesis that a major factor in the mechanism by which ICP0 influences virus infection is its ability to induce the degradation of specific cellular targets by interaction with the ubiquitin-proteasome pathway.
Figures
FIG. 1
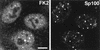
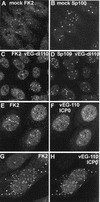
Distribution of conjugated ubiquitin in uninfected HEp-2 cells and its presence in some ND10. HEp-2 cells were simultaneously stained for conjugated ubiquitin with MAb FK2 (left panel) and for the ND10 component Sp100 with rabbit serum SpGH (right panel). A proportion of the major ND10 foci contain local accumulations of conjugated ubiquitin. Bar, 10 μm. The magnifications of most panels in other figures are similar, but in all cases the average width of a HEp-2 cell nucleus is 10 to 15 μm. The original colored images of this and all other multichannel figure panels may be viewed at
http://www.vir.gla.ac.uk/staff/everettrd/JVI832.shtml
.
FIG. 2
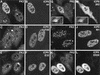
Expression of ICP0 in transfected cells leads to accumulation of colocalizing conjugated ubiquitin. HEp-2 cells were transfected with plasmids expressing wild-type and mutant forms of ICP0 and then stained to detect ICP0 with rabbit serum r191 (r95 for plasmids 110262 and E52X) and conjugated ubiquitin with MAb FK2. Each pair of panels shows the FK2 (left) and r191 (right) staining of the same field of view. (A and B) Wild-type ICP0. Note the difference in FK2 staining between the transfected (upper left) and untransfected (lower two) cells. Superimposition of the two images in the original data showed precise colocalization of the FK2 and ICP0 foci. (C and D) ICP0 RING finger deletion mutant FXE. (E and F) Exon 2 insertion mutant E32. The transfected cells are clearly distinguishable from the untransfected cells in the FK2 staining, but the result is not as striking as with the wild-type protein. (G and H) Exon 1 and exon 2 truncation mutant 110262 (ICP0R). The transfected cells have clearly altered FK2 staining patterns that in some cells had a punctate character (main panel) but was sometimes diffuse (inset). The 110262 staining pattern was generally diffuse throughout the whole cell. (I and J) USP7 binding-deficient point mutant M1. (K and L) C-terminal truncation mutant E52X. Although this mutant has a generally diffuse nuclear staining pattern, the transfected cells were easily distinguishable by examining the FK2 staining.
FIG. 3
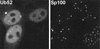
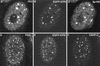
Distribution of an epitope-tagged ubiquitin precursor in transfected HEp-2 cells. Cells transfected with plasmid pCIQEUb52 were stained simultaneously with MAb MRGS to detect the tagged ubiquitin (left panel) and with SpGH to detect Sp100 (right panel). MAb MRGS gives no significant signal in the untransfected cells (upper and lower cells on the right of the left panel) and a diffuse nuclear distribution with some local accumulations which often coincide with ND10.
FIG. 4
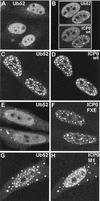
Cotransfection with plasmids expressing ICP0 and a ubiquitin precursor leads to colocalization of accumulations of the two proteins. HEp-2 cells were cotransfected with pCIQEUb52 (expressing the tagged ubiquitin precursor Ub52) and either vector alone (A) or plasmids expressing wild-type and mutant forms of ICP0 (B to H, as marked). (A) Tagged ubiquitin in singly transfected cells has a diffuse nuclear distribution with a number of small foci, some of which colocalize with ND10 (not shown). (B) Cells expressing ICP0 have a variable degree of punctate accumulation of ICP0; in cotransfected cells the ubiquitin precursor colocalizes with the punctate ICP0 foci. wt, wild type. (C to H) Each pair of images shows the tagged ubiquitin staining (left) and the ICP0 staining (right) of the same field of view. (C and D) Wild-type (wt) ICP0; (E and F) RING finger mutant FXE; (G and H) USP7 binding mutant M1.
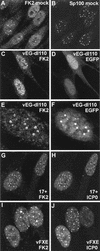
FIG. 5
Effect of HSV-1 infection on the distribution of conjugated ubiquitin in HEp-2 cells at 2 h postinfection. Each pair of images shows the same field of view with the conjugated ubiquitin (FK2) staining on the left (A, C, E, G) and either ND10 protein Sp100 (B and D) or ICP0 (F and H) staining on the right. (A and B) The distribution of conjugated ubiquitin in uninfected cells. A few foci are embedded in a generally diffuse distribution throughout the cell. Superimposition of the two images showed that some of the conjugated ubiquitin foci colocalize with ND10 (not shown). (C and D) Infection with ICP0 null mutant vEG-dl110 results in increased numbers of conjugated ubiquitin foci in the nucleus. The original triple-labeled image with the EGFP signal to identify infected cells is available on
http://www.vir.gla.ac.uk/staff/everettrd/JVI832.shtml
. (E and F) Infection with virus vEG-110 expressing wild-type ICP0 fused to EGFP results in induction of conjugated ubiquitin foci that colocalize with ICP0. Infected cells were easily detected on the basis of the FK2 staining alone. (G and H) When ICP0 accumulates in foci in the cytoplasm, it induces the formation of colocalizing conjugated ubiquitin here also.
FIG. 6
Triple colocalization of ICP0 and conjugated ubiquitin at ND10 and interphase centromeres in the early stages of infection. HEp-2 cells were infected with vEG-110 and stained for FK2 and either Sp100 or CENP-C as indicated. ICP0 was detected by the linked EGFP signal. All the major Sp100 foci (C) contain ICP0 (B), and all the ICP0 foci (B) react with MAb FK2 (A). In a certain proportion of cells, ICP0 also colocalizes with interphase centromeres, as shown here (D through F). The major ICP0 foci (E) are likely to be at ND10, but many of the minor foci are at centromeres (F). Of the 27 centromere foci visible in this confocal optical slice (F), at least 11 both contain ICP0 (E) and react with MAb FK2 (D). Because of the difficulty of reproducing the correct color balance of triple-colored images in printed journals, the single-channel data are shown here in greyscale. The original triple-colored images may be viewed at
http://www.vir.gla.ac.uk/staff/everettrd/JVI832.shtml
.
FIG. 7
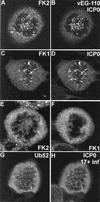
ICP0 induces the accumulation of conjugated ubiquitin at mitotic centromeres. (A and B) A mitotic cell infected with vEG110 and stained with MAb FK2. (C and D) A mitotic cell infected with HSV-1 strain 17+ costained with MAb FK1 and r191 to detect ICP0. (E and F) Corresponding uninfected controls. (G and H) HEp-2 cells singly transfected with pCIQEUb52 and later infected with HSV-1 strain 17+. This view shows a rare transfected cell that was infected in the late G2 phase of the cell cycle and has arrested in mitosis with the ICP0-induced localization of tagged ubiquitin at centromeres.
FIG. 8
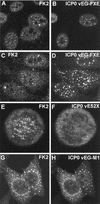
Effect of expression of mutant ICP0 proteins on the distribution of conjugated ubiquitin during HSV-1 infection of HEp-2 cells. The paired images show conjugated ubiquitin (left) and ICP0 fused to EGFP (right) except for the E52X results, which were obtained by staining for ICP0 with rabbit serum r95. (A and B) At early times of infection (2 h), RING finger mutant protein FXE accumulates at ND10 and there is variable colocalization with conjugated ubiquitin. (C and D) At later times of infection (4 h), foci of FXE protein in both nucleus and cytoplasm are less commonly associated with conjugated ubiquitin (compare with wild-type ICP0, Fig. 3I and J). (E and F) Although mutant ICP0 protein E52X inefficiently localizes at centromeres in mitotic cells, it clearly induces the accumulation of conjugated ubiquitin at these structures. (G and H) EGFP-linked ICP0 mutant M1 has a marked cytoplasmic punctate and perinuclear distribution that is mirrored by the induced colocalizing conjugated ubiquitin.
FIG. 9
Effect of expression of mutant ICP0 proteins on the distribution of conjugated ubiquitin during HSV-1 infection of HFL cells. The panel pairs show on the left the conjugated ubiquitin staining and on the right either Sp100 staining (B), EGFP (D and F), or EGFP-linked ICP0 (H and J). (A and B) The relationship between the distributions of conjugated ubiquitin and ND10 is similar to that in HEp-2 cells. (C and D) Infection of HFL cells by ICP0 null mutant vEG-dl110 for 2 h has a more marked effect on conjugated ubiquitin than in HEp-2 cells, with more prominent induction of nuclear foci, which are commonly associated with ND10 (not shown). EGFP itself forms foci in some cells, which can be associated with the conjugated ubiquitin foci. (E and F) At later times of infection, conjugated ubiquitin forms ringlike structures in some cells, and these frequently contain internal EGFP in vEG-dl110 infections. The faint clouds of EGFP in panel F correspond to replication compartments, which are generally not associated with the conjugated ubiquitin ring structures (which can occur in all mutant ICP0 virus infections but never in wild-type infections). (G to J) At 3 h postadsorption, EGFP-linked wild-type ICP0 is strongly associated with conjugated ubiquitin foci, but the RING finger deletion mutant FXE shows a variable degree of colocalization.
Similar articles
- Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro.
Boutell C, Sadis S, Everett RD. Boutell C, et al. J Virol. 2002 Jan;76(2):841-50. doi: 10.1128/jvi.76.2.841-850.2002. J Virol. 2002. PMID: 11752173 Free PMC article. - Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins.
Parkinson J, Everett RD. Parkinson J, et al. J Virol. 2000 Nov;74(21):10006-17. doi: 10.1128/jvi.74.21.10006-10017.2000. J Virol. 2000. PMID: 11024129 Free PMC article. - Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 induce the formation of colocalizing, conjugated ubiquitin.
Parkinson J, Everett RD. Parkinson J, et al. J Virol. 2001 Jun;75(11):5357-62. doi: 10.1128/JVI.75.11.5357-5362.2001. J Virol. 2001. PMID: 11333917 Free PMC article. - ICP0, a regulator of herpes simplex virus during lytic and latent infection.
Everett RD. Everett RD. Bioessays. 2000 Aug;22(8):761-70. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. Bioessays. 2000. PMID: 10918307 Review. - Regulation of alphaherpesvirus infections by the ICP0 family of proteins.
Boutell C, Everett RD. Boutell C, et al. J Gen Virol. 2013 Mar;94(Pt 3):465-481. doi: 10.1099/vir.0.048900-0. Epub 2012 Dec 12. J Gen Virol. 2013. PMID: 23239572 Review.
Cited by
- Recruitment of herpes simplex virus type 1 immediate-early protein ICP0 to the virus particle.
Maringer K, Elliott G. Maringer K, et al. J Virol. 2010 May;84(9):4682-96. doi: 10.1128/JVI.00126-10. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164220 Free PMC article. - Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro.
Boutell C, Sadis S, Everett RD. Boutell C, et al. J Virol. 2002 Jan;76(2):841-50. doi: 10.1128/jvi.76.2.841-850.2002. J Virol. 2002. PMID: 11752173 Free PMC article. - Herpes Simplex Virus 1 (HSV-1) Infected Cell Protein 0 (ICP0) Targets of Ubiquitination during Productive Infection of Primary Adult Sensory Neurons.
Harrell TL, Davido DJ, Bertke AS. Harrell TL, et al. Int J Mol Sci. 2023 Feb 2;24(3):2931. doi: 10.3390/ijms24032931. Int J Mol Sci. 2023. PMID: 36769256 Free PMC article. - Selective internalization of sodium channels in rat dorsal root ganglion neurons infected with herpes simplex virus-1.
Storey N, Latchman D, Bevan S. Storey N, et al. J Cell Biol. 2002 Sep 30;158(7):1251-62. doi: 10.1083/jcb.200204010. J Cell Biol. 2002. PMID: 12356869 Free PMC article. - Phosphorylation site mutations affect herpes simplex virus type 1 ICP0 function.
Davido DJ, von Zagorski WF, Lane WS, Schaffer PA. Davido DJ, et al. J Virol. 2005 Jan;79(2):1232-43. doi: 10.1128/JVI.79.2.1232-1243.2005. J Virol. 2005. PMID: 15613350 Free PMC article.
References
- Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. PIC1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. - PubMed
- Borden K L B. RING domains: master builders of molecular scaffolds? J Mol Biol. 2000;295:1103–1112. - PubMed
- Chelbi-Alix M K, de The H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene. 1999;18:935–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials