Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture - PubMed (original) (raw)
Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture
X p Chen et al. J Virol. 2000 Nov.
Abstract
Infected-cell protein 0 (ICP0), the product of the herpes simplex virus (HSV) immediate-early (IE) alpha0 gene, is a promiscuous transactivator of viral early (E) and late (L) gene expression. HSV mutants lacking ICP0 function are severely deficient in viral growth and protein synthesis, particularly at low multiplicities of infection. Early in the infectious process in vitro, ICP0 protein accumulates in distinct domains within the nucleus to form characteristic structures active in the transcription of viral genes. However, following infection of primary trigeminal ganglion cells in vitro with a recombinant HSV mutant that expresses only ICP0, we observed that ICP0 protein accumulated in the characteristic intranuclear distribution only in the nuclei of Schwann cells; neurons in the culture did not accumulate ICP0 despite expression of ICP0 RNA in those cells. The same phenomenon was observed in PC12 cells differentiated to assume a neuronal phenotype. In primary neurons in culture, the amount of ICP0 protein could be increased by pharmacologic inhibition of calcium-activated protease (calpain) activity or by inhibition of protein phosphatase 2B (calcineurin). The failure of ICP0 protein to accumulate in the nucleus of neurons suggests that one mechanism which may impair efficient replication of the virus in neurons, and thus favor the establishment of viral latency in those cells, may be found in the cell-specific processing of that IE gene product.
Figures
FIG. 1
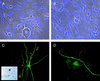
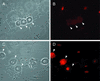
ICP0 protein accumulates in the nuclei of nonneuronal cells in dissociated TG cultures. Sixteen hours after infection with TOZ (MOI of 5), ICP0 is found in the nucleus of Schwann cells (arrowheads) but not neurons (arrow, identified by the characteristic appearance) in the cultures (phase contrast [A], fluorescence [B], and merged image [C]). At higher power, the immunostaining in the nucleus of the nonneuronal cells has the characteristic punctate appearance as described previously (D). ICP0 RNA can be detected in infected neurons by in situ hybridization with a digoxigenin-labeled riboprobe (inset, panel A).
FIG. 2
ICP0 does not accumulate in the nucleus of neurons in culture. The identity of the neurons was confirmed by immunostaining with an antibody to the neuron-specific antigen MAP2 (C and D). TOZ (A and C)- and mock (B and D)-infected cultures, 16 h postinfection, show ICP0 in a punctate pattern in the nucleus of Schwann cells but not of neurons in the infected cultures (A and C). X-Gal staining (inset, panel C) demonstrates that the majority of the cells in the culture, including neurons, were infected and expressed the lacZ transgene. Panels A and B are fused phase and fluorescent images of cultures counterstained with DAPI (4′,6′-diamidino-2-phenylindole).
FIG. 3
The pattern of accumulation of ICP0 after infection with replicating virus is similar to the pattern seen after infection with TOZ. Five hours after infection with wild-type KOS (MOI of 5), ICP0 is demonstrated by immunohistochemistry in the nucleus of nonneuronal cells (B) but is not seen in neurons (arrows, panels A and B). Wild-type KOS infected virtually all the cells in the culture, demonstrated by robust expression of HSV gC in neurons (D). Panels A and C are the phase micrographs corresponding to the fluorescent images in panels B and D.
FIG. 4
ICP0 RNA can be detected in approximately half of individual infected neurons by single-cell RT-PCR of infected neurons. Individual infected neurons identified by green fluorescence were isolated, and RT-PCR for β-actin was used to confirm RNA recovery. Approximately 50% of β-actin RNA-positive cells also showed ICP0 RNA by RT-PCR. Each lane represents the results of PCR amplification of cDNA obtained from a single identified infected cell in which β-actin was detected (upper panels). Results from four representative infected cells, two with detectable ICP0 RNA (lanes 1 and 2) and two without detectable ICP0 RNA (lanes 3 and 4), are shown. All of the uninfected cells (identified by the absence of fluorescence) in which β-actin RNA was detected were negative for ICP0 RNA (data not shown).
FIG. 5
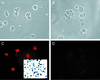
ICP0 protein accumulates in a punctate nuclear pattern in undifferentiated PC12 cells. Undifferentiated PC12 cells 16 h postinfection with TOZ (A and C) show the punctate nuclear pattern of ICP0 immunohistochemistry characteristic of nonneuronal cells. Virtually all the cells have been infected as demonstrated by X-Gal staining (inset, panel C). Mock-infected cultures are shown in panels B and D. (A and B) Phase microscopy. (C and D) Fluorescence.
FIG. 6
ICP0 fails to accumulate in the nucleus of differentiated PC12 cells. PC12 cells differentiated by exposure to NGF to assume a neuronal phenotype show only faint diffuse cytoplasmic immunostaining 16 h after infection with TOZ (C). Mock-infected cultures are shown in panels B and D. Virtually all the cells have been infected as demonstrated by X-Gal staining (inset, panel C). (A and B) Phase microscopy. (C and D) Fluorescence.
FIG. 7
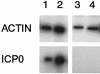
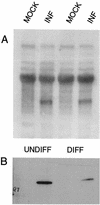
ICP0 RNA is expressed in both differentiated and undifferentiated PC12 cells, but the amount of protein is reduced in differentiated cells. Undifferentiated (UNDIFF) or differentiated (DIFF) PC12 cells were infected with TOZ at an MOI of 5, and RNA and protein were extracted 16 h after infection. Representative Northern (A) and Western (B) blots of RNA and protein from the infected (INF) and mock-infected PC12 cells are shown. The experiment was independently repeated four times, with similar results.
FIG. 8
Inhibition of calpain activity results in accumulation of ICP0 in neurons. Sixteen hours after infection of primary TG cultures with QOXHG at an MOI of 5, little immunoreactivity is seen in untreated neurons (arrowheads, panels A and B). Neurons (arrowheads) treated with PD 150606 to inhibit calpain activity show marked nuclear and cytoplasmic accumulation of immunoreactive ICP0 protein (C and D). Panels A and C show the phase micrographs corresponding to the fluorescent immunocytochemical images shown in panels B and D.
FIG. 9
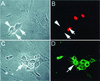
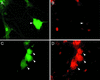
Inhibition of calcineurin activity results in accumulation of ICP0 in neurons. Sixteen hours after infection of primary TG cultures with recombinant QOZHG at an MOI of 5, no ICP0 immunoreactivity is seen in infected neurons (arrowheads). One neuron, identified by its characteristic morphology and identified as infected by the expression of GFP, is shown in panels A and B. A Schwann cell in the same culture is seen in the lower left of those panels. TG cultures treated with cyclosporine to inhibit calcineurin activity show marked accumulation of ICP0 in the nucleus and cytoplasm of infected neurons (arrowheads, panels C and D). Panels A and C are fluorescent micrographs of GFP expression. Panels B and D are cultures stained for ICP0.
Similar articles
- Accumulation and intranuclear distribution of unintegrated human immunodeficiency virus type 1 DNA.
Bell P, Montaner LJ, Maul GG. Bell P, et al. J Virol. 2001 Aug;75(16):7683-91. doi: 10.1128/JVI.75.16.7683-7691.2001. J Virol. 2001. PMID: 11462040 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Single-genome analysis reveals a heterogeneous association of the herpes simplex virus genome with H3K27me2 and the reader PHF20L1 following infection of human fibroblasts.
Francois AK, Rohani A, Loftus M, Dochnal S, Hrit J, McFarlane S, Whitford A, Lewis A, Krakowiak P, Boutell C, Rothbart SB, Kashatus D, Cliffe AR. Francois AK, et al. mBio. 2024 Apr 10;15(4):e0327823. doi: 10.1128/mbio.03278-23. Epub 2024 Feb 27. mBio. 2024. PMID: 38411116 Free PMC article. - Repression of the major immediate early promoter of human cytomegalovirus allows transcription from an alternate promoter.
Mason R, Bradley E, Wills M, Sinclair J, Reeves M. Mason R, et al. J Gen Virol. 2023 Sep;104(9). doi: 10.1099/jgv.0.001894. J Gen Virol. 2023. PMID: 37702591 - Pharmacological treatments in panic disorder in adults: a network meta-analysis.
Guaiana G, Meader N, Barbui C, Davies SJ, Furukawa TA, Imai H, Dias S, Caldwell DM, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A, Dawson S, Robertson L. Guaiana G, et al. Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
- Immobilized cobalt affinity chromatography provides a novel, efficient method for herpes simplex virus type 1 gene vector purification.
Jiang C, Wechuck JB, Goins WF, Krisky DM, Wolfe D, Ataai MM, Glorioso JC. Jiang C, et al. J Virol. 2004 Sep;78(17):8994-9006. doi: 10.1128/JVI.78.17.8994-9006.2004. J Virol. 2004. PMID: 15308696 Free PMC article. - Relaxed repression of herpes simplex virus type 1 genomes in Murine trigeminal neurons.
Terry-Allison T, Smith CA, DeLuca NA. Terry-Allison T, et al. J Virol. 2007 Nov;81(22):12394-405. doi: 10.1128/JVI.01068-07. Epub 2007 Sep 12. J Virol. 2007. PMID: 17855552 Free PMC article. - Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury.
Guo HL, Wolfe D, Epperly MW, Huang S, Liu K, Glorioso JC, Greenberger J, Blumberg D. Guo HL, et al. J Gastrointest Surg. 2003 Feb;7(2):229-35; discussion 235-6. doi: 10.1016/s1091-255x(02)00186-5. J Gastrointest Surg. 2003. PMID: 12600447 - Identification and characterization of a new E3 ubiquitin ligase in white spot syndrome virus involved in virus latency.
He F, Kwang J. He F, et al. Virol J. 2008 Dec 17;5:151. doi: 10.1186/1743-422X-5-151. Virol J. 2008. PMID: 19087357 Free PMC article. - Herpes simplex virus type 1 and bovine herpesvirus 1 latency.
Jones C. Jones C. Clin Microbiol Rev. 2003 Jan;16(1):79-95. doi: 10.1128/CMR.16.1.79-95.2003. Clin Microbiol Rev. 2003. PMID: 12525426 Free PMC article. Review.
References
- Aurelian L, Kokuba H, Smith C. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase. Vaccine. 1999;17:1951–1963. - PubMed
- Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. - PubMed
- Brondello J M, Pouyssegur J, McKenzie F R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources