Gene transfer to intact mesenteric arteries by electroporation - PubMed (original) (raw)
Gene transfer to intact mesenteric arteries by electroporation
J B Martin et al. J Vasc Res. 2000 Sep-Oct.
Abstract
The purpose of the present study was to develop a rapid, reproducible method of nonviral gene transfer to the intact vasculature. Male Sprague-Dawley rats were anesthetized, a midline abdominal incision was made and segmental branches of the superior mesenteric artery were dissected free of surrounding mesentery. A specially designed electroporation probe was placed around the neurovascular bundle and the electroporation chamber filled with a solution containing the firefly luciferase expressing plasmid (pCMV-Lux-DTS) or the green fluorescent protein expressing plasmid (pEGFP-N1). Vessels were electroporated with eight 10-ms pulses of 200 V/cm. Sixty seconds after electroporation, the DNA solution was removed, the intestine returned to the abdomen and the abdominal wall closed with suture and metal wound clips. Six hours to 5 days later, rats were sacrificed and electroporated vessels were recovered. Luciferase activity of the blood vessels was monitored. Gene expression was detected as early as 6 h postelectroporation, peaked at 1-3 days with levels up to 1 ng of reporter gene product per vessel segment and returned towards baseline by day 5. Histological analysis of blood vessel segments revealed green fluorescent protein-positive cells throughout the thickness of the vessel wall (endothelial cells to adventitia). Responses of electroporated vessels to vasoconstricting stimuli were indistinguishable from those of control vessels at either 2 or 40 days posttreatment. The results of this study provide evidence that electroporation is an effective means for introducing naked DNA into the blood vessel wall and form the basis for future studies on targeted gene therapy to the intact vasculature.
Copyright 2000 S. Karger AG, Basel
Figures
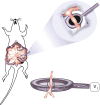
Figure 1. Cartoon of vascular electrode
Nickel wires were set 3 mm apart from each other, flanking a channel cut into the top of the electrode in which the artery to be electroporated is placed. The total volume of the electrode’s chamber is 55 μl.
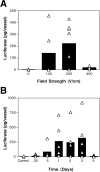
Figure 2. Voltage-dependency and time course of gene transfer and expression
Rat mesenteric arteries were placed into the electrode and incubated with pCMV-Lux-DTS (2 mg/ml) for several seconds prior to electroporation. (A) Square wave electroporations were performed using a train of 8 pulses of 10 millisecond duration each at field strengths of 0 to 400 V/cm. The arteries were removed 2 days later and luciferase activity was measured as described in Materials and Methods. (B) Vessels were electroporated with plasmid pCMV-Lux-DTS (2 mg/ml; 200 V/cm, 8 pulses, 10 millisecond duration). Arteries were removed at the indicated times and luciferase activities were determined. The bars represent the average expression of all arteries and the triangles represent the individual arteries. Vessels receiving no DNA are shown as “control”.
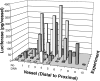
Figure 3. Luciferase expression in mesenteric arteries two days post-electroporation
Electroporations of pCMV-Lux-DTS (2 mg/ml) were performed using the standard settings (200 V/cm, 8 pulses, 10 millisecond duration). Control values represent arteries that received no DNA. In all experiments, arteries were electroporated in order from distal to proximal with respect to the ileocecal junction. Two days post-electroporation, vessels were removed and luciferase activities were measured as described in Materials and Methods. Differing numbers of arteries were electroporated in each animal, depending on the anatomy of the animals. Results from independent experiments are shown along the y-axis.
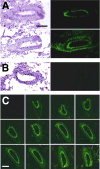
Figure 4. Distribution of gene expression in electroporated arteries
A mixture of pCMV-LUX-DTS and pEGFP (2mg/ml and 0.5 mg/ml, respectively) were transferred to mesenteric arteries with (A) or without (B) electroporation. After two days incubation, the arteries were removed and embedded for cryosectioning. Ten micron sections were prepared and GFP was visualized by fluorescence microscopy before the sections were stained with hemotoxylin and eosin. (C) GFP expression in successive 10 micron sections of an electroporated artery. All photographs were taken at an optical magnification of 25×, before computer analysis. Bars = 100 μm.
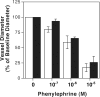
Figure 5. Vasoconstriction of electroporated arteries
Plasmid pCMV-LUX-DTS was transferred to rat mesenteric arteries by electroporation (8 pulses of 10 msec each; 200 V/cm). At 2 (A) or 40 (B) days post-transfer, the diameters of electroporated (filled bars) and untreated (“control”, open bars) vessels were determined by intravital microscopy using video calipers. Diameters are reported relative to baseline for the individual vessels, which ranged from 194 μm to 300 μm, n = 10 vessels. Average relative diameters ± standard deviations are shown for the vessels at each indicated concentration of phenylephrine. Vessels from 3 rats were used for measurements at day 2 and those from 2 rats were used for measurements at day 40.
Similar articles
- Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature.
Young JL, Benoit JN, Dean DA. Young JL, et al. Gene Ther. 2003 Aug;10(17):1465-70. doi: 10.1038/sj.gt.3302021. Gene Ther. 2003. PMID: 12900761 Free PMC article. - Electroporation as a method for high-level nonviral gene transfer to the lung.
Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Dean DA, et al. Gene Ther. 2003 Sep;10(18):1608-15. doi: 10.1038/sj.gt.3302053. Gene Ther. 2003. PMID: 12907953 Free PMC article. - Electroporation enhances reporter gene expression following delivery of naked plasmid DNA to the lung.
Pringle IA, McLachlan G, Collie DD, Sumner-Jones SG, Lawton AE, Tennant P, Baker A, Gordon C, Blundell R, Varathalingam A, Davies LA, Schmid RA, Cheng SH, Porteous DJ, Gill DR, Hyde SC. Pringle IA, et al. J Gene Med. 2007 May;9(5):369-80. doi: 10.1002/jgm.1026. J Gene Med. 2007. PMID: 17410613 - Electroporation-mediated ex vivo gene transfer into graft not requiring injection pressure in orthotopic liver transplantation.
Kobayashi S, Dono K, Takahara S, Isaka Y, Imai E, Zhenhui L, Nagano H, Tomoaki K, Umeshita K, Nakamori S, Sakon M, Monden M. Kobayashi S, et al. J Gene Med. 2003 Jun;5(6):510-7. doi: 10.1002/jgm.370. J Gene Med. 2003. PMID: 12797116 - [Experimental study of electroporation-mediated plasmid gene expression in skin and incisional wound].
Gao Z, Song N, Wu XL, Cao YL, Liu W. Gao Z, et al. Zhonghua Zheng Xing Wai Ke Za Zhi. 2008 Sep;24(5):390-3. Zhonghua Zheng Xing Wai Ke Za Zhi. 2008. PMID: 19119645 Chinese.
Cited by
- Nanosecond electroporation: another look.
Sundararajan R. Sundararajan R. Mol Biotechnol. 2009 Jan;41(1):69-82. doi: 10.1007/s12033-008-9107-y. Epub 2008 Sep 26. Mol Biotechnol. 2009. PMID: 18821065 Review. - Arbitrary Ca2+ regulation for endothelial nitric oxide, NFAT and NF-κB activities by an optogenetic approach.
Yamanaka T, Ueki T, Mase M, Inoue K. Yamanaka T, et al. Front Pharmacol. 2023 Jan 10;13:1076116. doi: 10.3389/fphar.2022.1076116. eCollection 2022. Front Pharmacol. 2023. PMID: 36703743 Free PMC article. - Electroporation-mediated delivery of catalytic oligodeoxynucleotides for manipulation of vascular gene expression.
Nunamaker EA, Zhang HY, Shirasawa Y, Benoit JN, Dean DA. Nunamaker EA, et al. Am J Physiol Heart Circ Physiol. 2003 Nov;285(5):H2240-7. doi: 10.1152/ajpheart.00350.2003. Epub 2003 Jul 24. Am J Physiol Heart Circ Physiol. 2003. PMID: 12881213 Free PMC article. - Electroporation-mediated gene delivery.
Young JL, Dean DA. Young JL, et al. Adv Genet. 2015;89:49-88. doi: 10.1016/bs.adgen.2014.10.003. Epub 2014 Dec 11. Adv Genet. 2015. PMID: 25620008 Free PMC article. - Gene transfer of the Na+,K+-ATPase beta1 subunit using electroporation increases lung liquid clearance.
Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GR, Yeldandi AV, Sznajder JI, Dean DA. Machado-Aranda D, et al. Am J Respir Crit Care Med. 2005 Feb 1;171(3):204-11. doi: 10.1164/rccm.200403-313OC. Epub 2004 Oct 29. Am J Respir Crit Care Med. 2005. PMID: 15516538 Free PMC article.
References
- Parker SE, Vahlsing HL, Serfilippi LM, Franklin CL, Doh SG, Gromkowski SH, Lew D, Manthorpe M, Norman J. Cancer gene therapy using plasmid DNA: safety evaluation in rodents and non-human primates [see comments] Hum Gene Ther. 1995;6:575–590. - PubMed
- Mir LM, Banoun H, Paoletti C. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: direct access to the cytosol. Exp Cell Res. 1988;175:15–25. - PubMed
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley & Sons; 1994.
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL059956/HL/NHLBI NIH HHS/United States
- DK51430/DK/NIDDK NIH HHS/United States
- R01 DK051430/DK/NIDDK NIH HHS/United States
- R01 HL059956-04/HL/NHLBI NIH HHS/United States
- HL38639/HL/NHLBI NIH HHS/United States
- HL59956/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources