G-Protein types involved in calcium channel inhibition at a presynaptic nerve terminal - PubMed (original) (raw)
G-Protein types involved in calcium channel inhibition at a presynaptic nerve terminal
R R Mirotznik et al. J Neurosci. 2000.
Abstract
The inhibition of presynaptic calcium channels via G-protein-dependent second messenger pathways is a key mechanism of transmitter release modulation. We used the calyx-type nerve terminal of the chick ciliary ganglion to examine which G-proteins are involved in the voltage-sensitive inhibition of presynaptic N-type calcium channels. Adenosine caused a prominent inhibition of the calcium current that was totally blocked by pretreatment with pertussis toxin (PTX), consistent with an exclusive involvement of G(o)/G(i) in the G-protein pathway. Immunocytochemistry was used to localize these G-protein types to the nerve terminal and its transmitter release face. We used two approaches to test for modulation by other G-protein types. First, we treated the terminals with ligands for a variety of G-protein-linked neurotransmitter receptor types that have been associated with different G-protein families. Although small inhibitory effects were observed, these could all be eliminated by PTX, indicating that in this terminal the G(i) family is the sole transmitter-induced G-protein inhibitory pathway. Second, we examined the kinetics of calcium channel inhibition by uncaging the nonselective and irreversible G-protein activator GTPgammaS, bypassing the receptors. A large fraction of the rapid GTPgammaS-induced inhibition persisted, consistent with a G(o)/G(i)-independent pathway. Immunocytochemistry identified G(q), G(11), G(12), and G(13) as potential PTX-insensitive second messengers at this terminal. Thus, our results suggest that whereas neurotransmitter-mediated calcium channel inhibition is mainly, and possibly exclusively, via G(o)/G(i), other rapid PTX-insensitive G-protein pathways exist that may involve novel, and perhaps transmitter-independent, activating mechanisms.
Figures
Fig. 1.
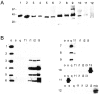
Characterization of G-protein antibodies.A, All antibodies gave an appropriate ∼40 kDa band for chick brain protein (10 μg/lane). Lane 1, Monoclonal anti-Go (Ab-1); 2, monoclonal anti-Go (Ab-2); 3, polyclonal anti-Go (J. K. Northup); 4, polyclonal anti-Gi3 (Calbiochem); 5, polyclonal anti-Gi1–3 (Santa Cruz Biotechnology); 6, polyclonal anti-Go+i3 (DuPont); 7, polyclonal anti-Gq/11 (DuPont); 8, polyclonal anti-Gq/11 (Santa Cruz Biotechnology); 9, polyclonal anti-Gq/11 (Calbiochem); 10, polyclonal anti-G12,13 (Gutkind); 11, polyclonal anti-Gs (DuPont); 12, polyclonal anti-Gz (Calbiochem). B, Blot of antibodies against the recombinant Gα subunits: Go, Gs, Gq, G11, Gi1, Gi2, Gi3, and G13, as well as a G-protein mixture that includes Gz.
Fig. 2.
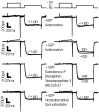
Modulation of chick ciliary ganglion presynaptic calcium current by neurotransmitter. The degree of current recruitment was determined using a double-trial protocol (see Materials and Methods) before, during, and after a puff of transmitter onto the terminal. Each panel shows before (left column) and during (right column) transmitter application in a single calyx nerve terminal. GTP (0.1 m
m
) was included in the internal solution in all experiments except in panel 1.Panel 1, Adenosine (10 μ
m
) in the absence of internal GTP. Panel 2, Adenosine (10 μ
m
) treatment. Panel 3, Treatment with a mix of substance P (0.5 μ
m
), bradykinin (1 μ
m
), neuropeptide Y (0.1 μ
m
), and BRL52537 (1 μ
m
). Panel 4, Treatment with a mix of noradrenaline (100 μ
m
) and somatostatin (10 μ
m
). −pp, Without prepulse; +pp, with prepulse.
Fig. 3.
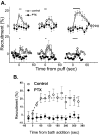
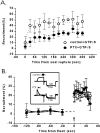
Effect of PTX pretreatment on adenosine-induced calcium current inhibition. A, Time course of calcium channel inhibition with puff application of transmitter (horizontal bar) with or without overnight PTX treatment. The effects of three consecutive trials given ∼1 min apart to each group of calyces are shown. _Top series,_Adenosine (10 μ
m
) treatment (control,n = 6; PTX, n = 14).Bottom series, Treatment with a cocktail of substance P (0.5 μ
m
), bradykinin (1 μ
m
), neuropeptide Y (0.1 μ
m
), and BRL52537 (1 μ
m
; control, n = 4; PTX,n = 3). B, Bath application of adenosine. Current inhibition was monitored after the addition (t = 0) of adenosine (0.2 m
m
) to control (open symbols, n = 6) or PTX-treated (filled symbols,n = 6) calyces. In both A and_B_ current inhibition is monitored by the percentage of prepulse recruitment.
Fig. 4.
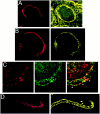
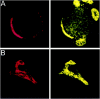
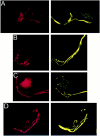
Localization of PTX-sensitive G-proteins. Gαo and Gαi localized to the membrane of the calyx presynaptic terminal. Calyx terminals are identified by SV2 (red) in the left panel of each pair except in C. A, Monoclonal antibodies (Ab-1 + Ab-2, yellow) localized Go to the membrane of calyx terminals in cryostat sections of whole ciliary ganglia. B, In dissociated preparations, monoclonal anti-Gαo (Ab-2) stained the membranes of presynaptic calyces with especially bright, patchy staining at the synaptic interface. C, Gi was localized by a multistaining approach. Go plus Gi were stained in red with polyclonal anti-Gαo (Northup;red; left panel) whereas Go was stained in green with monoclonal anti-Gαo(Ab-1 plus Ab-2; middle panel). Both labeled the calyx membrane. Superimposing the two stains (right panel) localizes Go by the costained regions (yellow). The distinct regions of red staining (e.g., asterisks) identify membrane regions with Gi but not Go. _D,_Fully isolated nerve terminals exhibited staining of the surface membrane with polyclonal anti-Gαo (Northup), confirming a presynaptic localization of Go/Gi.
Fig. 5.
Effect of PTX pretreatment on GTPγS-induced calcium channel inhibition. A, Intracellular infusion of GTPγS. Calcium currents were recorded every 10 sec after membrane rupture in each experiment, and the percentage of recruitment was averaged for 30 sec periods. Control, n = 5 experiments (open symbols); PTX, n = 6 (closed symbols). B, Flash photolysis of caged GTPγS with or without PTX pretreatment. Caged GTPγS was included in the internal solution, and ∼2 min after membrane seal rupture the caged nucleotide was released by a 200 msec flash (arrow). Immediately after, current trials were initiated (control, n = 7; PTX,n = 5). Insert, Representative current traces recorded 5–10 sec after the flash in a control and a PTX-treated terminal. −pp, Without prepulse; +pp, with prepulse. Symbols as in_A_.
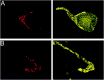
Fig. 6.
Localization of PTX-insensitive G-proteins: Gq/11. Gαq/11 (DuPont antibody) localized to the membrane and cytoplasm of the presynaptic terminal. Calyx terminals were identified by SV2 (red) in the_left panel_. Attached (A) and isolated (B) calyces had bright staining throughout the terminal that frequently colocalized with SV2.
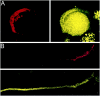
Fig. 7.
Localization of PTX-insensitive G-proteins: G12,13. Gα12,13 localized to both the membrane and cytoplasm of the calyx terminal. Calyx terminals are identified by SV2 (red) in the left panel. A, Attached calyces had spotty staining throughout the membrane and the cytoplasm. B, The staining remained in isolated calyces.
Fig. 8.
Localization of PTX-insensitive G-proteins: Gs. Gαs predominantly localized to the calyx cytoplasm. Calyx terminals are identified by SV2 (red) in each panel pair. A, Attached calyces were as stained as the postsynaptic neuron with reduced staining at the interface. B, Isolated calyces showed bright GαS staining along the axon that decreased at the terminal region.
Fig. 9.
Localization of PTX-insensitive G-proteins: Gz. Attached (A) and isolated (B) calyces had bright fibrous staining for Gz that coursed through the axon and partially into the terminal and was inversely correlated with SV2 (red, left panel). This staining pattern was suggestive of colocalization with the cytoskeleton. C, Tubulin α (red, left panel) stained predominantly near the calyx membrane and, hence, did not colocalize with the predominantly intracellular staining of Gz (yellow; right panel). D, Gz showed almost perfect colocalization (yellow, right panel) with the phosphorylated 200 kDa subunit of neurofilament protein (red, left panel).
Similar articles
- Selective blockade of P/Q-type calcium channels by the metabotropic glutamate receptor type 7 involves a phospholipase C pathway in neurons.
Perroy J, Prezeau L, De Waard M, Shigemoto R, Bockaert J, Fagni L. Perroy J, et al. J Neurosci. 2000 Nov 1;20(21):7896-904. doi: 10.1523/JNEUROSCI.20-21-07896.2000. J Neurosci. 2000. PMID: 11050109 Free PMC article. - Long splice variant N type calcium channels are clustered at presynaptic transmitter release sites without modular adaptor proteins.
Khanna R, Sun L, Li Q, Guo L, Stanley EF. Khanna R, et al. Neuroscience. 2006;138(4):1115-25. doi: 10.1016/j.neuroscience.2005.12.050. Epub 2006 Feb 13. Neuroscience. 2006. PMID: 16473471 - Voltage-dependent, pertussis toxin insensitive inhibition of calcium currents by histamine in bovine adrenal chromaffin cells.
Currie KP, Fox AP. Currie KP, et al. J Neurophysiol. 2000 Mar;83(3):1435-42. doi: 10.1152/jn.2000.83.3.1435. J Neurophysiol. 2000. PMID: 10712470 - Direct G protein modulation of Cav2 calcium channels.
Tedford HW, Zamponi GW. Tedford HW, et al. Pharmacol Rev. 2006 Dec;58(4):837-62. doi: 10.1124/pr.58.4.11. Pharmacol Rev. 2006. PMID: 17132857 Review. - G protein regulation of neuronal calcium channels: back to the future.
Proft J, Weiss N. Proft J, et al. Mol Pharmacol. 2015 Jun;87(6):890-906. doi: 10.1124/mol.114.096008. Epub 2014 Dec 30. Mol Pharmacol. 2015. PMID: 25549669 Review.
Cited by
- 'Fractional recovery' analysis of a presynaptic synaptotagmin 1-anchored endocytic protein complex.
Khanna R, Li Q, Stanley EF. Khanna R, et al. PLoS One. 2006 Dec 20;1(1):e67. doi: 10.1371/journal.pone.0000067. PLoS One. 2006. PMID: 17183698 Free PMC article. - Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony.
Kawasaki F, Collins SC, Ordway RW. Kawasaki F, et al. J Neurosci. 2002 Jul 15;22(14):5856-64. doi: 10.1523/JNEUROSCI.22-14-05856.2002. J Neurosci. 2002. PMID: 12122048 Free PMC article. - Spexin Enhances Bowel Movement through Activating L-type Voltage-dependent Calcium Channel via Galanin Receptor 2 in Mice.
Lin CY, Zhang M, Huang T, Yang LL, Fu HB, Zhao L, Zhong LL, Mu HX, Shi XK, Leung CF, Fan BM, Jiang M, Lu AP, Zhu LX, Bian ZX. Lin CY, et al. Sci Rep. 2015 Jul 10;5:12095. doi: 10.1038/srep12095. Sci Rep. 2015. PMID: 26160593 Free PMC article. - Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome.
Paulo JA, Brucker WJ, Hawrot E. Paulo JA, et al. J Proteome Res. 2009 Apr;8(4):1849-58. doi: 10.1021/pr800731z. J Proteome Res. 2009. PMID: 19714875 Free PMC article. - Inter-channel scaffolding of presynaptic CaV2.2 via the C terminal PDZ ligand domain.
Gardezi SR, Li Q, Stanley EF. Gardezi SR, et al. Biol Open. 2013 Apr 9;2(5):492-8. doi: 10.1242/bio.20134267. Print 2013 May 15. Biol Open. 2013. PMID: 23789098 Free PMC article.
References
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature (Lond) 1989;340:153–156. - PubMed
- Diversé-Pierluissi M, Dunlap K. Distinct, convergent second messenger pathways modulate neuronal calcium currents. Neuron. 1993;10:753–760. - PubMed
- Dolphin AC, Wooten JF, Scott RH, Trehtham DR. Photoactivation of intracellular guanosine triphosphate analogues reduces the amplitude and slows the kinetics of voltage-activated calcium channel currents in sensory neurons. Pflügers Arch. 1988;411:628–636. - PubMed
- Elmslie K. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources