p75 is important for axon growth and schwann cell migration during development - PubMed (original) (raw)
p75 is important for axon growth and schwann cell migration during development
C A Bentley et al. J Neurosci. 2000.
Abstract
Mice lacking the low-affinity neurotrophin receptor p75 have multiple peripheral neural deficits. Here we examined the developmental nature of these deficiencies. Peripheral axons in p75 -/- embryos were severely stunted and poorly arborized from embryonic day 11.5 (E11.5) to E14.5. In vitro, neurite outgrowth from the dorsal root ganglia was significantly decreased in the p75 -/- embryos at E12.5, suggesting that stunted axonal growth in the embryo may result in part from defects in neurite elongation. Additionally, Schwann cell marker S100beta immunoreactivity was decreased or absent along the growing axons of the ophthalmic branch from the trigeminal ganglia in p75 -/- embryos. Electron microscopy studies of the axons of the trigeminal ganglion at E13.5 revealed that in the p75 mutant embryo, nerve bundles were highly impaired and that coverage of the growing axons by Schwann cell cytoplasm was substantially reduced. In vitro, Schwann cell migration from the dorsal root ganglia was significantly decreased in the p75 -/- embryos at E12.5, suggesting that the lack of S100beta staining and Schwann cell coverage in the p75 mutant results from a deficit in Schwann cell migration. These results provide evidence that p75 is important in the developing embryo for regulating axon growth and arborization and for Schwann cell migration.
Figures
Fig. 1.
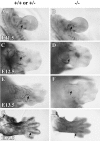
Whole-mount immunohistochemistry for neurofilament shows that innervation of the developing limbs is decreased in the p75 −/− embryos. Shown is a dorsal view of the forelimbs of controls (A, C, E,G) and p75 mutants (B, D,F, H) at ages E11.5 (A, B), E12.5 (C,D), E13.5 (E, F), and E14.5 (G, H).Arrows indicate immunoreactive axons projecting into the forelimbs. Photographs of whole mounts were taken using a dissecting microscope at 40× magnification.
Fig. 2.
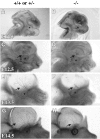
Neurofilament whole-mount immunohistochemistry of the skin from the head of developing embryos shows that axons of the ophthalmic branch of the TG are less extensive and complex in the p75 −/− embryos. Shown is a sagittal view of the head in the controls (A, C, E,G) and p75 mutants (B, D,F, H) at ages E11.5 (A, B), E12.5 (C,D), E13.5 (E, F), and E14.5 (G, H). The contralateral side of the head and the brain have been removed in_E_–H to increase transparency of the skin. Asterisks indicate location of the eye. Arrows point to axons of the ophthalmic branch. Photographs of whole mounts were taken using a dissecting microscope at 40× magnification.
Fig. 3.
In vitro neurite outgrowth from the DRG of control and p75 −/− embryos. Bar graph shows mean distance (mm) of neurite outgrowth and SEM in the presence of NGF (5 ng/ml) from the DRG of p75 +/+, +/−, and −/− embryos. Mean neurite outgrowth distance from the DRG of p75 −/− embryos is significantly lower (asterisk) than the outgrowth distances from the DRG of both p75 +/+ (p < 0.0001) and p75 +/− embryos (p < 0.0001). Representative micrographs showing vital dye staining of the DRG and extended neurites are overlaid with a line demonstrating measurement of neurite outgrowth distance.
Fig. 4.
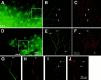
Whole-mount double immunohistochemistry using antibodies against S100β and TuJ1 to visualize the ophthalmic branch and associated Schwann cells of the TG in E14.5 embryos. Axons immunopositive for TuJ1 (green) are first shown at low-power magnification (A, D; scale bar, 500 μm). Asterisks in A and_D_ indicate where the TG axons emerge above the eye. The_white boxes_ in A and D_show regions visualized at high-power magnification in_B, C and E,F, respectively (scale bar, 100 μm). Axons in the p75 mutants (D_–_F) are severely stunted and less branched than those of the controls (A_–_C). S100β-staining (red) (C, F) appears to colocalize with TuJ1 staining (B,E). Arrows point out strong S100β (C) and TuJ1 (B) immunoreactivity in distal regions of axons in the control embryos. However, axons in the p75 −/− embryos have several regions of decreased or absent S100β staining (arrows in_E_, F). Higher-power magnification (scale bar, 50 μm) of axons in controls (G,H) shows robust staining of S100β (H), even at the distal tip (arrows in G, H) of the TuJ1-stained axons (G). Higher-power magnification of axons in p75 −/− embryos (I,J) shows that S100β staining (J) is absent at the distal tip of the TuJ1-positive (I) axons (top arrow in J, I) but can be seen more proximal to the TG (bottom arrow in_J_, I).
Fig. 5.
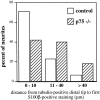
Analysis of S100β immunoreactivity in relation to the distal tips of tubulin-positive TG axons. Confocal images were used to measure the distance from the tip of the tubulin-positive axon to the nearest S100β-immunoreactive Schwann cell at E14.5. Three to five tubulin-positive tips were randomly chosen for measurement in control (n = 48) and mutant (_n_= 45) skin samples. The results of measurements are summarized.
Fig. 6.
Electron microscopy images of axons innervating the skin above the eye at E13.5 in control and p75 mutant embryos. Sections with the plane of the skin expose a roughly longitudinal view of the axon bundle with associated Schwann cells. Schwann cell nuclei (SCN) and axons are labeled. A, In micrograph from control embryo, axon bundle is organized and well defined. Schwann cell cytoplasm makes contact with and ensheaths the axon bundle (arrows). Cytoplasmic processes from Schwann cells also innervate the axon bundle (arrowheads). Scale bars (shown in A and B): 10 μm.B, In micrograph from p75 −/− embryos, the axon bundle is thin and disorganized. Schwann cell cytoplasmic processes make few contacts with the axon bundle (arrows), whereas much of the axon bundle is bare of Schwann cell cytoplasmic ensheathment (arrowheads). C, Higher-power magnification of box shown in B shows regions of the axon bundle in contact with Schwann cell cytoplasm (arrows) and regions of the axon bundle without Schwann cell cytoplasm ensheathment (arrowheads). Scale bar, 5 μm.
Fig. 7.
In vitro Schwann cell migration from the DRG of control and p75 −/− embryos. A,Bar graph shows mean distance (mm) of Schwann cell migration and SEM in 72 hr DRG cultures from p75 +/+, +/−, and −/− embryos. Migration of Schwann cells from the DRG of p75 −/− embryos is significantly lower (asterisk) than migration from both the DRG of p75 +/+ embryos (p < 0.001) and the DRG of p75 +/− embryos (p = 0.01). Representative micrographs showing vital dye staining of DRG and Schwann cells along the sciatic nerve are marked with a line demonstrating measurement of Schwann cell migration distance. B, Bar graph shows mean distance (mm) of Schwann cell migration and SEM without or with NGF (10 ng/ml) in 5 d cultures from control and p75 −/− embryos. Representative micrographs showing vital dye-stained Schwann cells along the sciatic nerve are marked with a_line_ demonstrating measurement of Schwann cell migration distance from original location of the DRG. Micrographs show that NGF-treated cultures from the DRG of both p75 +/− and p75 −/− embryos have many Schwann cells that are adhered to the glass coverslip.
Similar articles
- Schwann cell apoptosis in the postnatal axotomized sciatic nerve is mediated via NGF through the low-affinity neurotrophin receptor.
Petratos S, Butzkueven H, Shipham K, Cooper H, Bucci T, Reid K, Lopes E, Emery B, Cheema SS, Kilpatrick TJ. Petratos S, et al. J Neuropathol Exp Neurol. 2003 Apr;62(4):398-411. doi: 10.1093/jnen/62.4.398. J Neuropathol Exp Neurol. 2003. PMID: 12722832 - Schwann cell p75NTR prevents spontaneous sensory reinnervation of the adult spinal cord.
Scott AL, Ramer MS. Scott AL, et al. Brain. 2010 Feb;133(Pt 2):421-32. doi: 10.1093/brain/awp316. Epub 2010 Jan 3. Brain. 2010. PMID: 20047901 - A multifunctional neurotrophin with reduced affinity to p75NTR enhances transplanted Schwann cell survival and axon growth after spinal cord injury.
Enomoto M, Bunge MB, Tsoulfas P. Enomoto M, et al. Exp Neurol. 2013 Oct;248:170-82. doi: 10.1016/j.expneurol.2013.06.013. Epub 2013 Jun 20. Exp Neurol. 2013. PMID: 23792206 - [Immortalized adult rodent Schwann cells as useful tools for the study of peripheral nerve regeneration].
Sango K, Watabe K. Sango K, et al. Rinsho Shinkeigaku. 2013;53(11):1117-9. doi: 10.5692/clinicalneurol.53.1117. Rinsho Shinkeigaku. 2013. PMID: 24291897 Review. Japanese. - Estrogen receptor immunoreactivity in Schwann-like brain macroglia.
Gudiño-Cabrera G, Nieto-Sampedro M. Gudiño-Cabrera G, et al. J Neurobiol. 1999 Sep 15;40(4):458-70. doi: 10.1002/(sici)1097-4695(19990915)40:4<458::aid-neu4>3.0.co;2-9. J Neurobiol. 1999. PMID: 10453049 Review.
Cited by
- Multi-tasking by the p75 neurotrophin receptor: sortilin things out?
Bronfman FC, Fainzilber M. Bronfman FC, et al. EMBO Rep. 2004 Sep;5(9):867-71. doi: 10.1038/sj.embor.7400219. EMBO Rep. 2004. PMID: 15470383 Free PMC article. Review. - Molecular mechanisms in Schwann cell survival and death during peripheral nerve development, injury and disease.
Boyle K, Azari MF, Profyris C, Petratos S. Boyle K, et al. Neurotox Res. 2005;7(1-2):151-67. doi: 10.1007/BF03033784. Neurotox Res. 2005. PMID: 15639806 Review. - A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis?
Schirò G, Iacono S, Ragonese P, Aridon P, Salemi G, Balistreri CR. Schirò G, et al. Front Neurol. 2022 Jul 12;13:917527. doi: 10.3389/fneur.2022.917527. eCollection 2022. Front Neurol. 2022. PMID: 35911894 Free PMC article. Review. - The neurotrophin receptor p75 regulates gustatory axon branching and promotes innervation of the tongue during development.
Fei D, Huang T, Krimm RF. Fei D, et al. Neural Dev. 2014 Jun 24;9:15. doi: 10.1186/1749-8104-9-15. Neural Dev. 2014. PMID: 24961238 Free PMC article. - Chemically Induced Oncogenesis in the Peripheral Nervous System Is Suppressed in Congenic BDIX.BDIV-Mss1 and -Mss7 Rats.
Koelsch B, van den Berg L, Fischer C, Winzen-Reichert B, Kutritz A, Kindler-Röhrborn A. Koelsch B, et al. G3 (Bethesda). 2015 Nov 3;6(1):59-65. doi: 10.1534/g3.115.021170. G3 (Bethesda). 2015. PMID: 26530423 Free PMC article.
References
- Barker PA, Shooter EM. Disruption of NGF binding to the low-affinity neurotrophin receptor p75 reduces NGF binding to trkA on PC12 cells. Neuron. 1994;13:203–215. - PubMed
- Bergmann I, Priestley JV, McMahon SB, Bröcker E-B, Toyka KV, Koltzenburg M. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur J Neurosci. 1997;9:18–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials