Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study - PubMed (original) (raw)
Clinical Trial
Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study
J P Royet et al. J Neurosci. 2000.
Abstract
Neural correlates of responses to emotionally valenced olfactory, visual, and auditory stimuli were examined using positron emission tomography. Twelve volunteers were scanned using the water bolus method. For each sensory modality, regional cerebral blood flow (rCBF) during presentation of both pleasant and unpleasant stimuli was compared with that measured during presentation of neutral stimuli. During the emotionally valenced conditions, subjects performed forced-choice pleasant and unpleasant judgments. During the neutral conditions, subjects were asked to select at random one of a two key-press buttons. All stimulations were synchronized with inspiration, using an airflow olfactometer, to present the same number of stimuli for each sensory modality. A no-stimulation control condition was also performed in which no stimulus was presented. For all three sensory modalities, emotionally valenced stimuli led to increased rCBF in the orbitofrontal cortex, the temporal pole, and the superior frontal gyrus, in the left hemisphere. Emotionally valenced olfactory and visual but not auditory stimuli produced additional rCBF increases in the hypothalamus and the subcallosal gyrus. Only emotionally valenced olfactory stimuli induced bilateral rCBF increases in the amygdala. These findings suggest that pleasant and unpleasant emotional judgments recruit the same core network in the left hemisphere, regardless of the sensory modality. This core network is activated in addition to a number of circuits that are specific to individual sensory modalities. Finally, the data suggest a superior potency of emotionally valenced olfactory over visual and auditory stimuli in activating the amygdala.
Figures
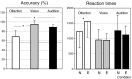
Fig. 1.
Left, Accuracy (%) of behavioral responses as a function of the three modalities and repetition (sequences 1 and 2). Right, Mean reaction times (in milliseconds) of subjects as a function of modality and emotional and neutral conditions.
Fig. 2.
The first four components of the PCA performed on the three activation conditions (modality, emotional, and repetition). Patterns of positive and negative covariance (eigenimages) of the four components that account for 86.5, 4.8, 3.2, and 1.4% of the variance, respectively. Bottom, Condition-dependent profiles (eigenvector) corresponding to positive and negative eigenimages of the first (A), second (B), third (C), and fourth (D) components. The first component discriminated activation patterns related to the auditory and olfactory conditions (positive eigenimage) and to the visual condition (negative eigenimage). The second component discriminated activation patterns related to the auditory (negative eigenimage) and olfactory (positive eigenimage) conditions. The third component discriminated activation patterns related to emotional (negative eigenimage) and neutral (positive eigenimage) conditions. The fourth component discriminated activation patterns related to the first (negative eigenimage) and the second (positive eigenimage) repetitions in each condition.Gray, Emotional condition; white, neutral condition.
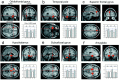
Fig. 3.
Sagittal, coronal, and transverse sections through the z maps on an anatomically normalized standard brain with areas activated in the three modalities in the emotional minus neutral conditions: in (a) the left inferior frontal gyrus, (b) the left temporal pole, (c) the left superior frontal gyrus; in olfaction and vision in (d) the hypothalamus and (e) the subcallosal gyrus; and (f) in olfaction in the emotional minus no-stimulation control conditions in both amygdalae. For each area activated, the plots show rCBF levels in the six activation conditions for this coordinate [5.35 ≤ z ≤ 6.27,p = 0.000 (corrected)].
Similar articles
- Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects.
Paradiso S, Johnson DL, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD. Paradiso S, et al. Am J Psychiatry. 1999 Oct;156(10):1618-29. doi: 10.1176/ajp.156.10.1618. Am J Psychiatry. 1999. PMID: 10518175 - Correlation between extraversion and regional cerebral blood flow in response to olfactory stimuli.
Vaidya JG, Paradiso S, Andreasen NC, Johnson DL, Boles Ponto LL, Hichwa RD. Vaidya JG, et al. Am J Psychiatry. 2007 Feb;164(2):339-41. doi: 10.1176/ajp.2007.164.2.339. Am J Psychiatry. 2007. PMID: 17267799 - Functional anatomy of perceptual and semantic processing for odors.
Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguière F, Comar D, Froment JC. Royet JP, et al. J Cogn Neurosci. 1999 Jan;11(1):94-109. doi: 10.1162/089892999563166. J Cogn Neurosci. 1999. PMID: 9950717 - Emotional Processing in the First 2 Years of Life: A Review of Near-Infrared Spectroscopy Studies.
Maria A, Shekhar S, Nissilä I, Kotilahti K, Huotilainen M, Karlsson L, Karlsson H, Tuulari JJ. Maria A, et al. J Neuroimaging. 2018 Sep;28(5):441-454. doi: 10.1111/jon.12529. Epub 2018 Jun 8. J Neuroimaging. 2018. PMID: 29883005 Free PMC article. Review.
Cited by
- Evaluation of Smell Function in Patients with Childhood Epilepsy with Centrotemporal Spikes at a Pediatric Neurology Clinic-A Case-Control Study.
Coşkun O, Karacabey BN, Ünal A, Paksoy S, Durak HN. Coşkun O, et al. J Clin Med. 2024 Oct 29;13(21):6474. doi: 10.3390/jcm13216474. J Clin Med. 2024. PMID: 39518613 Free PMC article. - Translation of monosynaptic circuits underlying amygdala fMRI neurofeedback training.
Trambaiolli L, Maffei C, Dann E, Biazoli C Jr, Bezgin G, Yendiki A, Haber S. Trambaiolli L, et al. Neuropsychopharmacology. 2024 Nov;49(12):1839-1850. doi: 10.1038/s41386-024-01944-w. Epub 2024 Aug 5. Neuropsychopharmacology. 2024. PMID: 39103495 Free PMC article. - Brain structural alterations associated with impulsiveness in male violent patients with schizophrenia.
Lu J, Gou N, Sun Q, Huang Y, Guo H, Han D, Zhou J, Wang X. Lu J, et al. BMC Psychiatry. 2024 Apr 15;24(1):281. doi: 10.1186/s12888-024-05721-3. BMC Psychiatry. 2024. PMID: 38622613 Free PMC article. - Large-Scale Estimation and Analysis of Web Users' Mood from Web Search Query and Mobile Sensor Data.
Sasaki W, Hamanaka S, Miyahara S, Tsubouchi K, Nakazawa J, Okoshi T. Sasaki W, et al. Big Data. 2024;12(3):191-209. doi: 10.1089/big.2022.0211. Epub 2023 Jun 2. Big Data. 2024. PMID: 37267209 - Temporopolar regions of the human brain.
Mesulam MM. Mesulam MM. Brain. 2023 Jan 5;146(1):20-41. doi: 10.1093/brain/awac339. Brain. 2023. PMID: 36331542 Free PMC article.
References
- Ahren GL, Schwartz GE. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23:745–755. - PubMed
- Angrilli A, Palomba D, Cantagallo A, Maietti A, Stegagno L. Emotional impairment after right orbitofrontal lesion in a patient without cognitive deficits. NeuroReport. 1999;10:1741–1746. - PubMed
- Barbas H, Ghasghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. - PubMed
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. - PubMed
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources