Persistent alterations in dendrites, spines, and dynorphinergic synapses in the nucleus accumbens shell of rats with neuroleptic-induced dyskinesias - PubMed (original) (raw)
Persistent alterations in dendrites, spines, and dynorphinergic synapses in the nucleus accumbens shell of rats with neuroleptic-induced dyskinesias
G E Meredith et al. J Neurosci. 2000.
Abstract
Chronic treatment of humans or experimental animals with classical neuroleptic drugs can lead to abnormal, tardive movements that persist long after the drugs are withdrawn. A role in these neuroleptic-induced dyskinesias may be played by a structural change in the shell of the nucleus accumbens where the opioid peptide dynorphin is upregulated in treated rats that show vacuous chewing movements (VCMs). The shell of the nucleus accumbens normally contains a dense plexus of dynorphinergic fibers especially in its caudomedial part. After 27 weeks of haloperidol administration and 18 weeks of withdrawal, the immunoreactive labeling of this plexus is intensified when compared with that after vehicle treatment. In addition, medium spiny neurons here show a significant increase in spine density, dendritic branching, and numbers of terminal segments. In the VCM-positive animals, the dendritic surface area is reduced, and dynorphin-positive terminals contact more spines and form more asymmetrical specializations than do those in animals without the syndrome (VCM-negative and vehicle-treated groups). Persistent, neuroleptic-induced oral dyskinesias could therefore be caused by incontrovertible alterations, involving terminal remodeling or sprouting, to the synaptic connectivity of the accumbal shell.
Figures
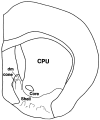
Fig. 1.
Diagram of a coronal section through the rat forebrain at a caudal level of the nucleus accumbens. Core and shell territories of the nucleus are labeled as are the dorsomedial (dm) and cone areas in the caudomedial shell.CPU, Caudate-putamen.
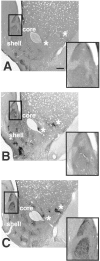
Fig. 2.
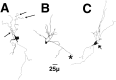
Light photomicrographs of sections through the neostriatum and caudal nucleus accumbens. Each section has been immunoreacted for dynorphin and is taken from a VEH-treated (A), VCM− (B), or VCM+ (C) rat. The caudomedial part of the shell of the nucleus accumbens (boxed area) has been enlarged in the inset of each micrograph. Note the intense immunoreactivity and sharp borders of the cone and dm (Fig. 1) areas of the shell in the VCM+ rat (C) as compared with those of the VCM− (B) and VEH-treated (A) animals. Note the homogeneous dynorphin immunoreactivity in the CPU. Asterisks mark small dynorphin-positive areas in the lateral core and shell of the nucleus. Note the increased immunoreactive intensity of these small regions in the VCM+ (C) rat as compared with that of the VCM− (B) and VEH-treated (A) animals. Scale bar: A, 500 μm (this scale bar is valid for B and C).
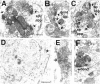
Fig. 3.
Electron micrographs taken from the shell of the nucleus accumbens in VCM+ (A–C), VCM− (D, E), and VEH-treated (F) rats. A, The dynorphin-immunoreactive terminal contacts two spines (arrows point to the active zone); one contact (right side) is perforated. B, A dynorphin-immunoreactive ending forms a perforated, asymmetrical synapse (4 perforations labeled with_arrows_) with a dendritic shaft (dend). Note the multivesicular body (mvb) within the terminal. C, A dense core vesicle (dcv) is marked with a_white_ arrow. D, A dynorphin-positive terminal (asterisk) makes a symmetrical contact with the soma in a VCM− rat. Note the round, nonindented nuclear envelope, indicating that this is a medium spiny neuron. E, terminal seen in D (asterisk).F, A dynorphin-positive bouton makes a symmetrical synaptic contact with a dendritic shaft in a VEH-treated animal. Scale bars: A, 0.5 μm (valid for B, C, and_F_); E, 0.1 μm; D, 2 μm. sp app, spine apparatus.
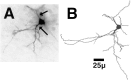
Fig. 4.
A, A typical neuron in the shell of the nucleus accumbens filled with biotinylated LY and reacted with DAB in a VEH-treated rat. Note the thickened, aspiny proximal dendritic segments (arrows). B, A reconstruction of the filled neuron pictured in A.
Fig. 5.
Reconstructions of typical, medium spiny neurons in the shell of the nucleus accumbens in a VEH-treated (A), VCM− (B), or VCM+ (C) rat. Note the tortuous distal dendritic segments (small arrows) in_A_ and the straight segments in B(asterisk) and in C. Note the higher density of the spines in B and C as compared with that in A and the spines on the proximal dendritic segment (large arrow) in_C_.
Fig. 6.
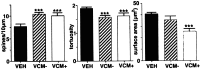
Bar graphs illustrate the significant differences between animal groups. Graphs show spine density (left), tortuosity (middle), and dendritic surface area (right) for VEH (filled bar), VCM− (hatched bar), and VCM+ (open bar) animals. The_left_ and middle graphs show that spine density is significantly (asterisks) increased and tortuosity is significantly reduced for both VCM+ and VCM− groups. The_right_ graph shows that dendritic segments in the VCM+ rats have significantly reduced surface areas as compared with those in the other two groups.
Fig. 7.
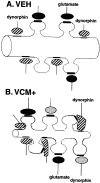
Schematic drawing of hypothetical changes that dynorphin-immunoreactive terminals undergo in animals that are VCM+.A, A sketch of a typical dendritic segment found in the shell of the nucleus accumbens of a VEH-treated rat. Dynorphin-positive endings (hatched) are shown contacting the dendritic shaft, and glutamatergic boutons (filled) are illustrated ending on spines. B, An illustration of a dendritic segment from a VCM+ rat. After 18 weeks of withdrawal from 27 weeks of haloperidol treatment, this dendrite has increased spine density and decreased surface area (see Fig. 6) when compared with that of the VEH-treated animal seen in A. In response to increased dynorphin production over the prolonged period, dynorphin-positive (hatched) axodendritic synapses change their shape and expand onto neighboring spines. Such growth would permit the receptor-bearing membrane located in the spine (Svingos et al., 1999) to come into close contact with a vesicle-bearing terminal, presumably rendering it functional. These dynorphinergic boutons would therefore form a new synapse on the spine, with the appropriate specialization, i.e., asymmetrical. Furthermore, new dynorphinergic terminals (dotted) may end on newly formed spines after axonal sprouting. Glutamate endings (solid) contact older spines. Regardless of the manner in which new synapses are formed, such structural modifications in dynorphin neurons and their synapses could alter the accumbal circuit in a dramatic and enduring manner.
Similar articles
- Pharmacological and neurochemical differences between acute and tardive vacuous chewing movements induced by haloperidol.
Egan MF, Hurd Y, Ferguson J, Bachus SE, Hamid EH, Hyde TM. Egan MF, et al. Psychopharmacology (Berl). 1996 Oct;127(4):337-45. doi: 10.1007/s002130050095. Psychopharmacology (Berl). 1996. PMID: 8923569 - Ultrastructural correlates of haloperidol-induced oral dyskinesias in rats: a study of unlabeled and enkephalin-labeled striatal terminals.
Roberts RC, Lapidus B. Roberts RC, et al. J Neural Transm (Vienna). 2003 Sep;110(9):961-75. doi: 10.1007/s00702-003-0013-y. J Neural Transm (Vienna). 2003. PMID: 12938022 - Correlation of vacuous chewing movements with morphological changes in rats following 1-year treatment with haloperidol.
Meshul CK, Andreassen OA, Allen C, Jørgensen HA. Meshul CK, et al. Psychopharmacology (Berl). 1996 Jun;125(3):238-47. doi: 10.1007/BF02247334. Psychopharmacology (Berl). 1996. PMID: 8815959 - Failure to down regulate NMDA receptors in the striatum and nucleus accumbens associated with neuroleptic-induced dyskinesia.
Hamid EH, Hyde TM, Baca SM, Egan MF. Hamid EH, et al. Brain Res. 1998 Jun 15;796(1-2):291-5. doi: 10.1016/s0006-8993(98)00196-6. Brain Res. 1998. PMID: 9689480 - Dynorphin-immunoreactive neurons in the rat nucleus accumbens: ultrastructure and synaptic input from terminals containing substance P and/or dynorphin.
Van Bockstaele EJ, Gracy KN, Pickel VM. Van Bockstaele EJ, et al. J Comp Neurol. 1995 Jan 2;351(1):117-33. doi: 10.1002/cne.903510111. J Comp Neurol. 1995. PMID: 7534773
Cited by
- Dendritic distributions of dopamine D1 receptors in the rat nucleus accumbens are synergistically affected by startle-evoking auditory stimulation and apomorphine.
Hara Y, Pickel VM. Hara Y, et al. Neuroscience. 2007 Jun 8;146(4):1593-605. doi: 10.1016/j.neuroscience.2007.04.005. Epub 2007 May 9. Neuroscience. 2007. PMID: 17490822 Free PMC article. - Prescription of Anticholinergics in Tardive Syndromes: A "Dual Center" Survey among Psychiatrists.
Cutino A, Bhidayasiri R, Colosimo C. Cutino A, et al. Parkinsons Dis. 2020 Nov 22;2020:8870945. doi: 10.1155/2020/8870945. eCollection 2020. Parkinsons Dis. 2020. PMID: 33299541 Free PMC article. - The structural basis for mapping behavior onto the ventral striatum and its subdivisions.
Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. Meredith GE, et al. Brain Struct Funct. 2008 Sep;213(1-2):17-27. doi: 10.1007/s00429-008-0175-3. Epub 2008 Feb 7. Brain Struct Funct. 2008. PMID: 18256852 Free PMC article. Review. - Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens.
Pickel VM, Chan J, Kearn CS, Mackie K. Pickel VM, et al. J Comp Neurol. 2006 Mar 20;495(3):299-313. doi: 10.1002/cne.20881. J Comp Neurol. 2006. PMID: 16440297 Free PMC article. - Relevance of animal models to human tardive dyskinesia.
Blanchet PJ, Parent MT, Rompré PH, Lévesque D. Blanchet PJ, et al. Behav Brain Funct. 2012 Mar 9;8:12. doi: 10.1186/1744-9081-8-12. Behav Brain Funct. 2012. PMID: 22404856 Free PMC article. Review.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid and corticopetal components of the substantia innominata. Neuroscience. 1988;27:1–39. - PubMed
- Bardo MT, Hammer RP., Jr Autoradiographic localization of dopamine D1 and D2 receptors in the rat nucleus accumbens: resistance to differential rearing conditions. Neuroscience. 1991;45:281–290. - PubMed
- Benes FM, Paskevich PA, Domesick VB. Haloperidol-induced plasticity of axon terminals in rat substantia nigra. Science. 1983;221:969–971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources