Yeast Krr1p physically and functionally interacts with a novel essential Kri1p, and both proteins are required for 40S ribosome biogenesis in the nucleolus - PubMed (original) (raw)
Yeast Krr1p physically and functionally interacts with a novel essential Kri1p, and both proteins are required for 40S ribosome biogenesis in the nucleolus
T Sasaki et al. Mol Cell Biol. 2000 Nov.
Abstract
Using a two-hybrid screening with TOM1, a putative ubiquitin-ligase gene of Saccharomyces cerevisiae, we isolated KRR1, a homologue of human HRB2 (for human immunodeficiency virus type 1 Rev-binding protein 2). To characterize the gene function, we constructed temperature-sensitive krr1 mutants and isolated two multicopy suppressors. One suppressor is RPS14A, encoding a 40S ribosomal protein. The C-terminal-truncated rpS14p, which was reported to have diminished binding activity to 18S rRNA, failed to suppress the krr1 mutant. The other suppressor is a novel gene, KRI1 (for KRR1 interacting protein; YNL308c). KRI1 is essential for viability, and Kri1p is localized to the nucleolus. We constructed a galactose-dependent kri1 strain by placing KRI1 under control of the GAL1 promoter, so that expression of KRI1 was shut off when transferring the culture to glucose medium. Polysome and 40S ribosome fractions were severely decreased in the krr1 mutant and Kri1p-depleted cells. Pulse-chase analysis of newly synthesized rRNAs demonstrated that 18S rRNA is not produced in either mutant. However, wild-type levels of 25S rRNA are made in either mutant. Northern analysis revealed that the steady-state levels of 18S rRNA and 20S pre-rRNAs were reduced in both mutants. Precursors for 18S rRNA were detected but probably very unstable in both mutants. A myc-tagged Kri1p coimmunoprecipitated with a hemagglutinin-tagged Krr1p. Furthermore, the krr1 mutant protein was defective in its interaction with Kri1p. These data lead us to conclude that Krr1p physically and functionally interacts with Kri1p to form a complex which is required for 40S ribosome biogenesis in the nucleolus.
Figures
FIG. 1
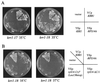
A high dose of RPS14A or KRI1 suppresses the temperature sensitivity of krr1 mutants. (A) A plasmid YEplac181 (YEp-vector), pTS1034 (YCp-KRR1), pTS1501 (YEp-RPS14A), or pTS1601 (YEp-KRI1) was introduced into the krr1 mutant strains YTS094 (krr1-17) and YTS095 (krr1-18). Transformants were streaked on SD-Leu medium which was incubated at 35°C for 3 days. (B) The strain YTS095 (krr1-18) harboring YEplac181 (YEp-vector), pTS1034 (YCp-KRR1), pTS1501 (YEp-RPS14A), pTS1506 (YEp-rpS14_-Δ_C11), or pTS1508 (rpS14-CryR) was streaked on SD-Leu medium which was incubated at 35 or 37°C for 3 days.
FIG. 2
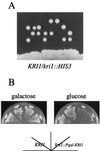
The novel gene KRI1 is essential. (A) KRI1 is essential for viability. A heterozygous diploid strain, YTS100 (KRI1/kri1::HIS3) was sporulated, and the tetrads were dissected on a YPD plate, which was incubated at 25°C for 4 days. (B) The effect of depletion of Kri1p. Strains YTS097A (kri1::Pgal-KRI1) and W303-1A (KRI1) were streaked on galactose or glucose medium and incubated at 25°C for 3 or 2 days, respectively.
FIG. 3
Krr1p and Kri1p are nucleolar proteins. Strain YTS029-4A (Krr1-5HAp) or YTS076 (Kri1-2HAp), each harboring pTS068 to express Nop1-GFPp, was stained with anti-HA antibody and Cy3-conjugated secondary antibody (B, Krr1-HAp; E, Kri1-HAp). Nop1-GFPp was used as a nucleolar marker (C and F). DNA was detected by 4′, 6′-diamino-2-phenylindole (DAPI) staining (A and D).
FIG. 4
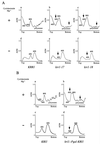
The level of 40S ribosome subunits decreases in both krr1 and kri1 mutants under the restrictive conditions. (A) Cultures of YTS055 (KRR1 [a and d]), temperature-sensitive krr1 mutant YTS094 (krr1-17 [b and e]), and YTS095 (krr1-18 [c and f]) were grown at 37°C for 6 h. The cell lysates were prepared with (+ [a, b, and c]) or without (− [d, e, and f]) cycloheximide and Mg2+ and were centrifuged through 7 to 47% sucrose density gradients. (B) The wild-type strain W303-1A (KRI1 [a and c]) and the glucose-repressible kri1 strain YTS097A (kri1::Pgal-KRI1 [b and d]) were transferred to glucose medium and grown at 25°C for 24 h. Extracts were prepared and analyzed as described for panel A. Arrows indicate aberrant patterns observed in the krr1 and kri1 mutants.
FIG. 5
Both Krr1p and Kri1p are required for the synthesis of 18S rRNA. (A) Cultures of YTS055 (KRR1), YTS094 (krr1-17), and YTS095 (krr1-18) were grown in SD medium without methionine at 37°C for 4 h. Cells were pulse-labeled for 2 min with [_methyl_-3H]methionine (P2), which was chased for 5 min (C5) or 10 min (C10) with an excess of cold methionine. A total of 60,000 cpm was loaded on each lane. (B) Wild-type strain W303-1A (KRI1) and the glucose-repressible kri1 strain YTS097A (kri1::Pgal-KRI1) were grown in glucose medium at 25°C for 24 h. Cells were pulse-labeled and chased as described for panel A. A total of 30,000 cpm (KRI1) or 240,000 cpm (kri1::Pgal-KRI1) was loaded on each lane.
FIG. 6
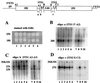
The steady-state level of rRNAs in both krr1 and kri1 mutants. (A through D) Cultures of YTS055 (KRR1; lanes 1 and 2), YTS094 (krr1-17; lanes 3 and 4) and YTS095 (krr1-18; lanes 5 and 6) were grown at 25°C (lanes 1, 3, and 5) or 37°C (lanes 2, 4, and 6) for 4 h. (A) RNA was prepared and electrophoresed through a 1.2% agarose–formaldehyde gel which was stained with ethidium bromide (EtBr). (B through D) For a Kri1p-depletion experiment, W303-1A (KRI1; lanes 7 and 8) and the glucose-repressible kri1 strain YTS097A (kri1::Pgal-KRI1; lanes 9 and 10) were grown in galactose (lanes 7 and 9) or glucose medium (lanes 8 and 10) at 25°C for 24 h. The agarose gels were subjected to Northern blotting, using oligonucleotides complementary to ITS1 5′-A2 (oligo a) (B), ITS1 A2–A3 (oligo b) (C), and ITS2 E-C2 (oligo c) (D). The positions of various rRNAs are indicated.
FIG. 7
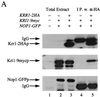
Association of Krr1p with Kri1p in vivo. (A) Strains of W303-1A (Krr1p and Kri1p; lane 1), YTS084 (Krr1p and Kri1-9mycp; lanes 2 and 4) containing pTS068 (Nop1p-GFP) and YTS085 (Krr1-2HAp and Kri1-9mycp; lanes 3 and 5) harboring pTS068 (Nop1p-GFP) were grown at 26°C to mid-log phase. The cell lysates were prepared and immunoprecipitated with anti-HA antibody, and the bound proteins were subjected to Western blotting with anti-HA, anti-myc, or anti-GFP antibody. + or − signifies the presence or absence, respectively, of the indicated tagged proteins. (B) The mutant Krr1p impaired the association with Kri1p. Cultures of YTS084 (Krr1p and Kri1-9mycp; lanes 1 and 2), YTS085 (Krr1-2HAp and Kri1-9mycp; lanes 3 and 4), YTS089 (Krr1-17-2HAp and Kri1-9mycp; lanes 5 and 6) and YTS090 (Krr1-18-2HAp and Kri1-9mycp; lanes 7 and 8) were grown at 26°C (lanes 1, 3, 5, and 7) or 37°C (lanes 2, 4, 6, and 8) for 4 h. Immunoprecipitation was performed as described in panel A. Breakdown products of Kri1-9mycp are indicated by a bracket with an asterisk.
FIG. 7
Association of Krr1p with Kri1p in vivo. (A) Strains of W303-1A (Krr1p and Kri1p; lane 1), YTS084 (Krr1p and Kri1-9mycp; lanes 2 and 4) containing pTS068 (Nop1p-GFP) and YTS085 (Krr1-2HAp and Kri1-9mycp; lanes 3 and 5) harboring pTS068 (Nop1p-GFP) were grown at 26°C to mid-log phase. The cell lysates were prepared and immunoprecipitated with anti-HA antibody, and the bound proteins were subjected to Western blotting with anti-HA, anti-myc, or anti-GFP antibody. + or − signifies the presence or absence, respectively, of the indicated tagged proteins. (B) The mutant Krr1p impaired the association with Kri1p. Cultures of YTS084 (Krr1p and Kri1-9mycp; lanes 1 and 2), YTS085 (Krr1-2HAp and Kri1-9mycp; lanes 3 and 4), YTS089 (Krr1-17-2HAp and Kri1-9mycp; lanes 5 and 6) and YTS090 (Krr1-18-2HAp and Kri1-9mycp; lanes 7 and 8) were grown at 26°C (lanes 1, 3, 5, and 7) or 37°C (lanes 2, 4, 6, and 8) for 4 h. Immunoprecipitation was performed as described in panel A. Breakdown products of Kri1-9mycp are indicated by a bracket with an asterisk.
Similar articles
- Functional and physical interactions of Krr1p, a Saccharomyces cerevisiae nucleolar protein.
Gromadka R, Karkusiewicz I, Rempoła B, Rytka J. Gromadka R, et al. Acta Biochim Pol. 2004;51(1):173-87. Acta Biochim Pol. 2004. PMID: 15094838 - The Saccharomyces cerevisiae TIF6 gene encoding translation initiation factor 6 is required for 60S ribosomal subunit biogenesis.
Basu U, Si K, Warner JR, Maitra U. Basu U, et al. Mol Cell Biol. 2001 Mar;21(5):1453-62. doi: 10.1128/MCB.21.5.1453-1462.2001. Mol Cell Biol. 2001. PMID: 11238882 Free PMC article. - Ribosome Biogenesis: A Central Player in Cancer Metastasis and Therapeutic Resistance.
Elhamamsy AR, Metge BJ, Alsheikh HA, Shevde LA, Samant RS. Elhamamsy AR, et al. Cancer Res. 2022 Jul 5;82(13):2344-2353. doi: 10.1158/0008-5472.CAN-21-4087. Cancer Res. 2022. PMID: 35303060 Free PMC article. Review. - Human diseases of the SSU processome.
Sondalle SB, Baserga SJ. Sondalle SB, et al. Biochim Biophys Acta. 2014 Jun;1842(6):758-64. doi: 10.1016/j.bbadis.2013.11.004. Epub 2013 Nov 12. Biochim Biophys Acta. 2014. PMID: 24240090 Free PMC article. Review.
Cited by
- Stepwise and dynamic assembly of the earliest precursors of small ribosomal subunits in yeast.
Zhang L, Wu C, Cai G, Chen S, Ye K. Zhang L, et al. Genes Dev. 2016 Mar 15;30(6):718-32. doi: 10.1101/gad.274688.115. Genes Dev. 2016. PMID: 26980190 Free PMC article. - The small-subunit processome is a ribosome assembly intermediate.
Bernstein KA, Gallagher JE, Mitchell BM, Granneman S, Baserga SJ. Bernstein KA, et al. Eukaryot Cell. 2004 Dec;3(6):1619-26. doi: 10.1128/EC.3.6.1619-1626.2004. Eukaryot Cell. 2004. PMID: 15590835 Free PMC article. - Genetic screening for modifiers of the DREF pathway in Drosophila melanogaster: identification and characterization of HP6 as a novel target of DREF.
Ida H, Suzusho N, Suyari O, Yoshida H, Ohno K, Hirose F, Itoh M, Yamaguchi M. Ida H, et al. Nucleic Acids Res. 2009 Apr;37(5):1423-37. doi: 10.1093/nar/gkn1068. Epub 2009 Jan 9. Nucleic Acids Res. 2009. PMID: 19136464 Free PMC article. - Npa1p, a component of very early pre-60S ribosomal particles, associates with a subset of small nucleolar RNPs required for peptidyl transferase center modification.
Dez C, Froment C, Noaillac-Depeyre J, Monsarrat B, Caizergues-Ferrer M, Henry Y. Dez C, et al. Mol Cell Biol. 2004 Jul;24(14):6324-37. doi: 10.1128/MCB.24.14.6324-6337.2004. Mol Cell Biol. 2004. PMID: 15226434 Free PMC article. - Mutation of kri1l causes definitive hematopoiesis failure via PERK-dependent excessive autophagy induction.
Jia XE, Ma K, Xu T, Gao L, Wu S, Fu C, Zhang W, Wang Z, Liu K, Dong M, Jing C, Ren C, Dong Z, Chen Y, Jin Y, Huang Q, Chang X, Deng M, Li L, Luo L, Zhu J, Dang Y, Chang HC, Zon LI, Zhou Y, Chen S, Pan W. Jia XE, et al. Cell Res. 2015 Aug;25(8):946-62. doi: 10.1038/cr.2015.81. Epub 2015 Jul 3. Cell Res. 2015. PMID: 26138676 Free PMC article.
References
- Burns N, Grimwade B, Ross-Macdonald P B, Choi E Y, Finberg K, Roeder G S, Snyder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials