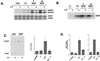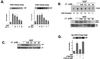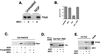Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1 - PubMed (original) (raw)
Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1
R D York et al. Mol Cell Biol. 2000 Nov.
Abstract
Neurotrophins promote multiple actions on neuronal cells including cell survival and differentiation. The best-studied neurotrophin, nerve growth factor (NGF), is a major survival factor in sympathetic and sensory neurons and promotes differentiation in a well-studied model system, PC12 cells. To mediate these actions, NGF binds to the TrkA receptor to trigger intracellular signaling cascades. Two kinases whose activities mediate these processes include the mitogen-activated protein (MAP) kinase (or extracellular signal-regulated kinase [ERK]) and phosphoinositide 3-kinase (PI3-K). To examine potential interactions between the ERK and PI3-K pathways, we studied the requirement of PI3-K for NGF activation of the ERK signaling cascade in dorsal root ganglion cells and PC12 cells. We show that PI3-K is required for TrkA internalization and participates in NGF signaling to ERKs via distinct actions on the small G proteins Ras and Rap1. In PC12 cells, NGF activates Ras and Rap1 to elicit the rapid and sustained activation of ERKs respectively. We show here that Rap1 activation requires both TrkA internalization and PI3-K, whereas Ras activation requires neither TrkA internalization nor PI3-K. Both inhibitors of PI3-K and inhibitors of endocytosis prevent GTP loading of Rap1 and block sustained ERK activation by NGF. PI3-K and endocytosis may also regulate ERK signaling at a second site downstream of Ras, since both rapid ERK activation and the Ras-dependent activation of the MAP kinase kinase kinase B-Raf are blocked by inhibition of either PI3-K or endocytosis. The results of this study suggest that PI3-K may be required for the signals initiated by TrkA internalization and demonstrate that specific endocytic events may distinguish ERK signaling via Rap1 and Ras.
Figures
FIG. 1
Role of PI3-K in neuronal ERK activation. (A) ERK activity was assessed by immunostaining fixed cells with a pERK1/2 antibody. Adult rat DRG cultures were treated for 20 min with either 50 ng of NGF per ml (left) or 10 μM forskolin (right) in the presence or absence of LY (20 μM), a specific PI3-K inhibitor. For each pair of photos, light-field micrographs are shown on the left and fluorescence micrographs showing pERK1/2 immunoreactivity are shown on the right. The signal intensity in neurons was quantitated for each treatment condition using NIH Image. Histograms represent the distribution of fluorescent intensities as a function of frequency (n = 100 cells). LY completely abolished ERK activation by NGF but had no effect on forskolin-stimulated ERKs. (B) Immunofluorescence localization of B-Raf expression in adult rat DRG cultures. Note that expression is detected only in neurons. (C) Biochemical examination of B-Raf in cultured DRG neurons. B-Raf activity was measured by immune complex kinase assay using anti-B-Raf antisera and recombinant MEK-1 as a substrate. A representative gel is shown (n = 3). (D) Average values from three experiments as shown in panel C, presented as mean fold activation with standard errors indicated by the error bars.
FIG. 2
Requirement of PI3-K for ERK activation by NGF in PC12 cells. PC12 cells were left untreated (Untr. and U) or treated with forskolin plus IBMX (F/I) for 20 min or NGF for either 5 min (5′) or 40 min (40′), as indicated. (A) Phosphorylation of ERKs was used as a measure of ERK activation in cell lysates monitored by Western blotting using pERK1/2 antibodies. (B) ERK activation was measured in cell lysates by immune complex kinase assay using an anti-ERK2 antibody and MBP as a substrate. In both assays, LY blocked ERK activation by NGF but not by forskolin. (C) (left) PI3-K assay showing blockade of NGF-induced PI3-K activity by LY (20 μM). (Right) Average values from three assays are presented as fold activation with standard errors indicated by the error bars. (D) LY inhibition of the transcription factor CREB, an ERK-dependent target of NGF. Following transfection with GAL4-CREB (2 μg) and Gal-luciferase (2 μg), cells were left untreated or treated with NGF, LY, or the MEK inhibitor PD98059 (PD) (40 μM) as indicated. CREB-dependent transcription was monitored by luciferase assay and reported as fold increase in luciferase activity. CREB activation by NGF was sensitive to LY as well as PD.
FIG. 3
PI3-K inhibitors block NGF activation of PI3-K and ERK. (A) Dose-dependent action of LY and wortmannin on ERK activation by NGF (15 min). Cells were treated with NGF and either LY (0.05, 0.5, 5.0, and 50 μM) or wortmannin (Wort) (0.01, 0.05, 0.1, 0.5, and 1 μM), as indicated. ERK activation was measured in cell lysates by immune complex kinase assay using an anti-ERK2 antibody and MBP as a substrate. A representative gel is shown (upper panels) along with the average values from three experiments, with standard errors indicated by the error bars (bar graphs). ERK activity is presented as fold activation over basal levels. (B) Dose dependency of LY's inhibition of PI3-K activity. Cells were treated with NGF and LY (0.05, 0.5, 5.0, and 50 μM), and PI3-K activity was measured as described in Materials and Methods (upper panel). Fold increase in PI3-K activity is shown below the top panel. Lower panels show the action of LY on NGF-induced phosphorylation of ERK in the same cell lysates, as measured by Western blotting using pERK antibodies (middle panel). The expression of total ERKs is shown as a loading control (bottom panel). (C) Dose dependency of LY inhibition of pAkt. Cells were treated with NGF and LY (0.05, 0.5, 5.0, and 50 μM), lysates were prepared, and the levels of pAkt were visualized by Western blotting (upper panel). The expression of total Akt is shown (bottom panel). (D) Inhibition of NGF activation of ERK by PTEN. PC12 cells were cotransfected with myc-ERK2 and wild-type (wt) PTEN or a mutated PTEN [PTEN (G129E)] which lacks lipid phosphatase activity. myc-ERK2 activation was measured in cell lysates by immune complex kinase assay using an anti-myc antibody (9E10) and MBP as a substrate. A representative gel is shown (upper panel) along with the average values from three experiments, with standard errors indicated by the error bars (bar graph). myc-ERK2 activity is presented as fold over basal levels.
FIG. 4
LY inhibition of Rap1 and B-Raf activation. (A) LY inhibition of NGF-induced B-Raf activation. PC12 cells were left untreated (Untr.) or treated with NGF and LY as indicated, and B-Raf activity was measured by immune complex kinase assay using anti-B-Raf antisera and recombinant MEK-1 as a substrate. 5′ and 40′, 5 and 40 min, respectively. (B) Western blot showing expression of Rap1 and Ras in PC12 cells and DRG cells, as indicated. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gels. (C) LY inhibition of NGF-induced Rap1 activation. PC12 cells were either left untreated or treated with NGF, forskolin plus IBMX (Forsk./IBMX) and LY as indicated. Lysates were incubated with GST-RalGDS and precipitated with glutathione beads. The amount of active Rap1 bound to the beads was measured by SDS-PAGE followed by Western blotting using anti-Rap1 antibodies. (D) Lack of LY inhibition of NGF-induced Ras activation. Lysates were either left untreated (Untr.) or treated as indicated. Lysates were prepared and incubated with GST-Raf1-RBD and precipitated with glutathione beads. The amount of active Ras bound to the beads was measured by SDS-PAGE followed by Western blotting using anti-Ras antibodies.
FIG. 5
Requirement of PI3-K for Ras coupling to downstream kinases. (A) Independence of Rap1 for early activation of ERK by NGF. PC12 cells were transfected with myc-ERK2 in the absence (−) or presence (+) of cotransfected Rap1GAP1 (RapGAP), and treated with NGF for the indicated times (in minutes). myc-ERK2 activation was measured in cell lysates by immune complex kinase assay using an anti-myc antibody and MBP as a substrate. The amount of myc-ERK2 within each lysate is shown in the lower panels. A representative gel is shown (n = 3). (B) PC12 cells were cotransfected with cDNA encoding constitutively active Ras (RasV12), constitutively active Raf (BXBRaf), or constitutively active MEK (caMEK), along with Flag-ERK2, and treated with LY or cotransfected with PTEN, as indicated. Flag-ERK2 activity was assessed by anti-Flag immunoprecipitation followed by pERK Western blotting. The amount of total ERK is shown in the lower panel. (C) Recruitment of B-Raf to Ras upon NGF treatment. PC12 cells were transfected with His-Ras and treated with NGF in the absence or presence of LY as indicated. His-Ras and associated proteins were precipitated from lysates using nickel-NTA agarose, associated proteins were resolved by SDS-PAGE, and B-Raf was detected by Western blotting. The position of B-Raf (95 kDa) is shown. (D) NGF stimulation of Ras-associated B-Raf kinase activity. PC12 cells were transfected with His-Ras and/or PTEN and treated with NGF or LY as indicated. B-Raf activity within His-Ras eluates was measured by immune complex kinase assay using MEK-1 (MEK) as a substrate.
FIG. 6
Facilitation of NGF-induced TrkA internalization by PI3-K. (A) PC12 cells were left untreated (Untr.) or treated with NGF in the presence (+) or absence (−) of LY, as indicated. Cell surface proteins were biotinylated as described in Materials and Methods. Biotinylated proteins were recovered using UltraLink Immobilized NeutrAvidin (Pierce), and the amount of TrkA recovered was assessed by Western blotting. A representative blot with the position of TrkA is shown. (B) Bar graph showing the averages from three biotinylation experiments as in panel A. The data are presented as percentages of maximal stimulation, with standard errors indicated by the error bars. (C) Phosphorylation of Akt is completely blocked by LY. Parallel plates of PC12 cells were treated as in panel A and examined for pAkt expression by Western blotting. The position of pAkt (upper panel) is shown, along with total Akt (lower panel) as a loading control. (D) Phosphorylation of TrkA is not affected by LY. Cells were treated with LY and NGF as indicated, and lysates were examined for pTrkA expression by Western blotting. A representative blot is shown, and the position of pTrkA is indicated (n = 3).
FIG. 7
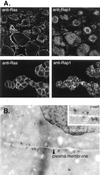
Subcellular localization of Ras and Rap1 in PC12 cells. (A) Immunofluorescence detection of Ras and Rap1. DRG cells (upper panels) or PC12 cells (lower panels) were fixed and incubated with monoclonal antibodies to Ras and polyclonal antibodies to Rap1. Anti-Ras (left panels) and Anti-Rap1 (right panels) were visualized by confocal microscopy. (B) Immunoelectron microscopy of Rap1. Using immunogold electron microscopy and anti-Rap1 antibodies, immunogold particles were detected on sections of fixed PC12 cells. Two adjacent PC12 cells are shown with their plasma membranes indicated. The nucleus of one cell can be seen. Gold particles are clustered within vesicular structures. Magnification, ×48,000. The insert shows vesicular structures with gold particles.
FIG. 8
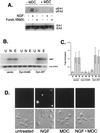
MDC inhibition of NGF signaling to ERK. (A) MDC blocks TrkA internalization. PC12 cells were left untreated or treated with NGF in the presence (+) or absence (−) of a 10-min pretreatment with MDC as indicated. Cell surface proteins were biotinylated and precipitated as described in the legend to Fig. 6, and the amount of TrkA recovered was assessed by Western blotting. A representative blot with the position of TrkA is shown. (B) Bar graph showing the averages from three independent experiments as shown in panel A, with standard errors indicated by the error bars. (C) Inhibition of NGF-induced Rap1 activation by MDC. PC12 cells were treated with NGF or forskolin plus IBMX (Forsk/IBMX) for 10 min in the presence or absence of MDC as indicated. Rap1 activation was measured using Gst-RalGDS “pull-down” as described in the legend to Fig. 4. The position of Rap1 is shown. (D) Lack of inhibition of NGF-induced Ras activation by MDC. Cells were treated with NGF and MDC as indicated, and Ras activation was measured using Gst-Raf1-RBD pull-down as described in the legend to Fig. 4. The position of Ras in PC12 lysates is shown. (E) Inhibition of Ras-dependent recruitment and activation of B-Raf by MDC. Cells were transfected with His-Ras and treated with NGF in the presence or absence of MDC as indicated. His-Ras and associated proteins were precipitated from lysates using nickel-NTA agarose, and associated proteins were eluted from His-Ras as described in Materials and Methods. Eluates were split and assayed for associated B-Raf protein by Western blotting (upper panel) or for associated B-Raf kinase activity (lower panel), as measured by immune complex assay using MEK1 as a substrate (n = 3). The position of MEK1 is shown. The position of B-Raf within PC12 lysates is shown in the upper panel (lysate).
FIG. 9
Requirement of endocytosis for ERK activation by NGF in PC12 and DRG cells. (A) PC12 cells were treated with NGF or forskolin plus IBMX (Forsk./IBMX) for 10 min in the presence (+) or absence (−) of MDC, as indicated. Activation of ERK was measured in cell lysates by Western blotting using pERK1/2 antibodies. The positions of pERKs (pErk1 and pErk2) are shown. The amount of total ERKs in each lysate is shown in the lower panel. (B) PC12 cells were transfected with myc-ERK2 in the presence or absence of either wild-type dynamin (Dyn-WT) or a mutated dynamin (Dyn-K44E), and cells were treated with NGF (N) or EGF (E) or left untreated (U). myc-ERK2 activation was measured in cell lysates by immune complex kinase assay using an anti-myc antibody and MBP as a substrate. A representative gel is shown (upper panel) and the amount of myc-ERK2 within each lysate is shown below (lower panel). (C) Data from three independent experiments as performed in panel B are represented as fold activation, with standard errors indicated by the error bars. (D) Adult rat DRG cultures were treated for 20 min with NGF in the presence or absence of MDC. Fluorescence micrograph showing pERK1/2 immunoreactivity (upper panels) and light-field micrographs (lower panels) are shown.
FIG. 10
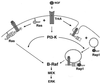
Model of NGF signaling to ERKs. In PC12 cells, NGF activates Ras and Rap1 to mediate the rapid and sustained activation of ERKs, respectively. Both TrkA internalization and Rap1 activation require PI3-K. Clathrin-mediated endocytosis is also required for Rap1 activation and the sustained activation of ERK. In contrast, Ras activation by NGF is independent of both PI3-K activity and endocytosis. This may reflect the distinct localizations of Ras at the plasma membrane and Rap1 within endosomal compartments. PI3-K-dependent endocytosis may regulate ERK signaling at a second site downstream of Ras, since both rapid ERK activation by NGF and Ras activation of B-Raf are blocked by both PI3-K inhibitors and inhibitors of endocytosis.
Similar articles
- Sustained activation of M-Ras induced by nerve growth factor is essential for neuronal differentiation of PC12 cells.
Sun P, Watanabe H, Takano K, Yokoyama T, Fujisawa J, Endo T. Sun P, et al. Genes Cells. 2006 Sep;11(9):1097-113. doi: 10.1111/j.1365-2443.2006.01002.x. Genes Cells. 2006. PMID: 16923128 - Molecular dissection of TrkA signal transduction pathways mediating differentiation in human neuroblastoma cells.
Eggert A, Ikegaki N, Liu X, Chou TT, Lee VM, Trojanowski JQ, Brodeur GM. Eggert A, et al. Oncogene. 2000 Apr 13;19(16):2043-51. doi: 10.1038/sj.onc.1203518. Oncogene. 2000. PMID: 10803465 - A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway.
Wu C, Ramirez A, Cui B, Ding J, Delcroix JD, Valletta JS, Liu JJ, Yang Y, Chu S, Mobley WC. Wu C, et al. Traffic. 2007 Nov;8(11):1503-20. doi: 10.1111/j.1600-0854.2007.00636.x. Epub 2007 Sep 6. Traffic. 2007. PMID: 17822405 - The Mystery of Rap1 Suppression of Oncogenic Ras.
Nussinov R, Jang H, Zhang M, Tsai CJ, Sablina AA. Nussinov R, et al. Trends Cancer. 2020 May;6(5):369-379. doi: 10.1016/j.trecan.2020.02.002. Epub 2020 Mar 2. Trends Cancer. 2020. PMID: 32249186 Free PMC article. Review. - Rit subfamily small GTPases: regulators in neuronal differentiation and survival.
Shi GX, Cai W, Andres DA. Shi GX, et al. Cell Signal. 2013 Oct;25(10):2060-8. doi: 10.1016/j.cellsig.2013.06.002. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770287 Free PMC article. Review.
Cited by
- Role of phosphatidylinositol-4,5-bisphosphate 3-kinase signaling in vesicular trafficking.
Bhattacharya S, McElhanon KE, Gushchina LV, Weisleder N. Bhattacharya S, et al. Life Sci. 2016 Dec 15;167:39-45. doi: 10.1016/j.lfs.2016.10.018. Epub 2016 Oct 17. Life Sci. 2016. PMID: 27760304 Free PMC article. Review. - Ginsenosides: changing the basic hallmarks of cancer cells to achieve the purpose of treating breast cancer.
Jiang RY, Fang ZR, Zhang HP, Xu JY, Zhu JY, Chen KY, Wang W, Jiang X, Wang XJ. Jiang RY, et al. Chin Med. 2023 Sep 25;18(1):125. doi: 10.1186/s13020-023-00822-9. Chin Med. 2023. PMID: 37749560 Free PMC article. Review. - Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1.
Schmitt JM, Stork PJ. Schmitt JM, et al. Mol Cell Biol. 2001 Jun;21(11):3671-83. doi: 10.1128/MCB.21.11.3671-3683.2001. Mol Cell Biol. 2001. PMID: 11340161 Free PMC article. - APP regulates NGF receptor trafficking and NGF-mediated neuronal differentiation and survival.
Zhang YW, Chen Y, Liu Y, Zhao Y, Liao FF, Xu H. Zhang YW, et al. PLoS One. 2013 Nov 1;8(11):e80571. doi: 10.1371/journal.pone.0080571. eCollection 2013. PLoS One. 2013. PMID: 24224055 Free PMC article. - The role of rab proteins in neuronal cells and in the trafficking of neurotrophin receptors.
Bucci C, Alifano P, Cogli L. Bucci C, et al. Membranes (Basel). 2014 Oct 6;4(4):642-77. doi: 10.3390/membranes4040642. Membranes (Basel). 2014. PMID: 25295627 Free PMC article. Review.
References
- Avruch J, Zhang X-F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous