Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II - PubMed (original) (raw)
Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II
S J Wicks et al. Mol Cell Biol. 2000 Nov.
Abstract
Members of the transforming growth factor beta (TGF-beta) family transduce signals through Smad proteins. Smad signaling can be regulated by the Ras/Erk/mitogen-activated protein pathway in response to receptor tyrosine kinase activation and the gamma interferon pathway and also by the functional interaction of Smad2 with Ca(2+)-calmodulin. Here we report that Smad-TGF-beta-dependent transcriptional responses are prevented by expression of a constitutively activated Ca(2+)-calmodulin-dependent protein kinase II (Cam kinase II). Smad2 is a target substrate for Cam kinase II in vitro at serine-110, -240, and -260. Cam kinase II induces in vivo phosphorylation of Smad2 and Smad4 and, to a lesser extent, Smad3. A phosphopeptide antiserum raised against Smad2 phosphoserine-240 reacted with Smad2 in vivo when coexpressed with Cam kinase II and by activation of the platelet-derived growth factor receptor, the epidermal growth factor receptor, HER2 (c-erbB2), and the TGF-beta receptor. Furthermore, Cam kinase II blocked nuclear accumulation of a Smad2 and induced Smad2-Smad4 hetero-oligomerization independently of TGF-beta receptor activation, while preventing TGF-beta-dependent Smad2-Smad3 interactions. These findings provide a novel cross-talk mechanism by which Ca(2+)-dependent kinases activated downstream of multiple growth factor receptors antagonize cell responses to TGF-beta.
Figures
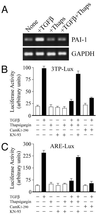
FIG. 1
Inhibition of TGF-β-dependent transcriptional responses by Cam kinase II. (A) The expression of the PAI-1 gene was monitored in Cos-1 cells by semiquantitative PCR as described in Materials and Methods. In some instances cells were pretreated with 1 μM thapsigargin in DMSO and then stimulated with 5 ng of TGF-β per ml for 15 h. Expression of GAPDH was monitored as an internal control. Cos-1 cells were transiently transfected with 2 μg of pCMV1-Cam kinase II1–290 or empty pCMV1, together with 2 μg of 3TP-lux reporter plasmid (B), or 2 μg of ARE-lux reporter plasmid (C). In some instances cells were pretreated with 1 μM thapsigargin in DMSO and then stimulated with 5 ng of TGF-β per ml for 15 h (black bars) as indicated. Some cells were also pretreated for 1 h with the specific Cam kinase II inhibitor, KN-93, at a concentration of 10 μM. Control cells, in the absence of TGF-β, were also treated with either KN-93 or KN-93 and thapsigargin.
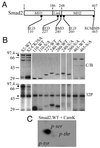
FIG. 2
In vitro mapping of Cam kinase II phosphorylation sites in Smad2. (A) Schematic representation of Smad2 highlighting the linker region and the highly conserved MH1 and MH2 domains. The location and sequence of putative RXXS and SXD consensus Cam kinase II sites are indicated. (B) Smad2-GST fusion proteins were prepared and phosphorylated in vitro with Cam kinase II as described in Materials and Methods. Samples were separated by SDS–12.5% PAGE, and gels were either stained with Coomassie blue (C/B) or dried and exposed to X-ray film (32P). The migration of autophosphorylated Cam kinase II (∗) and full-length Smad2-GST (⧫) are indicated. (C) Separation of phosphoamino acids in GST-S2-WT (residues 1 to 467) phosphorylated with Cam kinase II. GST-S2-WT was separated by SDS–12.5% PAGE, blotted onto PVDF membrane, and acid hydrolyzed, and phosphoamino acids were separated by thin-layer chromatography (TLC). The migration of the following phosphoamino acids is indicated: phosphoserine (p-ser), phosphothreonine (p-thr), and phosphotyrosine (p-tyr).
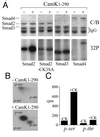
FIG. 3
Smads are substrates for Cam kinase II in vivo. HEK-293 cells were transfected with HA-Smad2, HA-Smad2-CK3SA, HA-Smad3, or FLAG-Smad4 in the presence or absence of Cam kinase II1–290 and metabolically labeled with [32P]-orthophosphate. Cell lysates were immunoprecipitated either with anti-flag M2 antibody or anti-HA antibody. (A) Immunoprecipitates were separated by SDS–10% PAGE and stained with Coomassie blue (C/B) to detect total Smad protein, or phosphoproteins were visualized by autoradiography (32P). (B) HA-Smad2 immunoprecipitates were blotted onto PVDF, HA-Smad2 proteins acid were hydrolyzed, and phosphoamino acids were separated by TLC. The migration of the following phosphoamino acids is indicated: phosphoserine (p-ser), phosphothreonine (p-thr), and phosphotyrosine (p-tyr). (C) Radioactive phosphoamino acids were scraped from the TLC plate and quantitated by Cerenkov counting.
FIG. 4
Phosphorylation of serine-240 occurs downstream of multiple growth factor receptors. (A) HA-tagged Smad2 was expressed in HEK-293 cells in the presence or absence of Cam kinase II1–290 and immunoprecipitated with anti-HA, and samples were probed by Western blotting against anti-HA or anti-PS240 antibodies. Equivalent expression levels were monitored in whole lysates by anti-HA Western blotting. Expression of Cam kinase II1–290 was confirmed by using specific antisera (data not shown). HA-tagged Smad2 was also expressed in HEK-293 cells in the presence or absence of the constitutively active type I TGF-β receptor (TGFβRI*), the human EGF receptor (EGFR), or chimeric receptors comprising the extracellular domain of the EGF receptor connected to the respective PDGF (EP-R) or HER2 (HER-1/2) intracellular domains. The EGF receptor and chimeric receptors were activated with 100 ng of EGF per ml for 30 min immediately prior to lysis. Samples were immunoprecipitated with anti-HA, and samples were probed by Western blotting against anti-HA or anti-PS240 antibodies. Equivalent expression levels were monitored by anti-HA blotting, and the expression of receptors was confirmed with specific antisera (not shown). In some instances, cells were pretreated for 1 h with the specific Cam kinase II inhibitor, KN-93, at a concentration of 10 μM. (B) HepG2 cells were stimulated for 30 min with the indicated growth factors. In some instances, cells were pretreated for 1 h with the specific Cam kinase II inhibitor, KN-93, at a concentration of 10 μM. Cells were lysed, and samples were probed by Western blotting against Smad2 or PS-240.
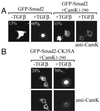
FIG. 5
Cam kinase II regulates the subcellular localization of Smad2-GFP. Smad2-GFP fusion proteins were expressed in Cos-1 cells, grown in 0.5% fetal calf containing medium, and stimulated 48 h posttransfection with TGF-β for 15 h. The percentage of Smad2 localized to the nucleus was determined by counting 100 immunofluorescence-positive cells and is denoted in the top left corner of each image. Cos-1 cells were also transfected with Smad2-GFP, together with Cam kinase II1–290, and the percentage of nuclear-positive cells was assessed as described above only in cells expressing both the Smad and Cam kinase. Cotransfection was confirmed with primary Cam kinase II mouse monoclonal antibody (anti-Cam kinase II) and secondary TRITC-conjugated goat anti-mouse antibody. (A) Wild-type Smad2. (B) Smad2-CK3SA.
FIG. 6
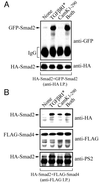
Cam kinase II induces Smad2-Smad4 interactions independently of TGF-β receptor activation. (A) HEK-293 cells were transfected with N-terminally tagged HA-Smad2 and GFP-Smad2 in the presence or absence of the constitutively active type I TGF-β receptor (TGFβR*), constitutively active Cam kinase II (Cam kinase II1–290), or both (TGFβR* plus Cam kinase II1–290). Cell lysates were immunoprecipitated with anti-HA, and samples were probed by immunoblotting against anti-HA or anti-GFP antibodies. Expression of the TGF-β receptor and Cam kinase II was confirmed using specific antisera (data not shown). (B) HEK-293 cells were transfected with N-terminally tagged HA-Smad2 and C-terminally tagged FLAG-Smad4 in the presence or absence of the constitutively active type I TGF-β receptor (TGFβR*), constitutively active Cam kinase II (Cam kinase II1–290), or both (TGFβR* plus Cam kinase II1–290). Cell lysates were immunoprecipitated with anti-FLAG, and samples were probed by immunoblotting against anti-HA, anti-FLAG, or anti-PS2 antibodies. Expression of the TGF-β receptor and Cam kinase II was confirmed by using specific antisera (data not shown).
FIG. 7
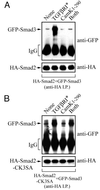
TGF-β-dependent Smad2-Smad3 interactions are prevented by Cam kinase II. HEK-293 cells were transfected with N-terminally tagged HA-Smad2 (A) or HA-Smad2-CK3SA (B), together with GFP-Smad3, in the presence or absence of the constitutively active type I TGF-β receptor (TGFβR*), constitutively active Cam kinase II (Cam kinase II1–290), or both (TGFβR* plus Cam kinase II1–290). Cell lysates were immunoprecipitated with anti-HA, and samples were probed by immunoblotting against anti-HA or anti-GFP antibodies. Expression of the TGF-β receptor and Cam kinase II was confirmed using specific antisera (data not shown).
Similar articles
- TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4.
Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P. Nakao A, et al. EMBO J. 1997 Sep 1;16(17):5353-62. doi: 10.1093/emboj/16.17.5353. EMBO J. 1997. PMID: 9311995 Free PMC article. - The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells.
Poncelet AC, de Caestecker MP, Schnaper HW. Poncelet AC, et al. Kidney Int. 1999 Oct;56(4):1354-65. doi: 10.1046/j.1523-1755.1999.00680.x. Kidney Int. 1999. PMID: 10504488 - Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation.
Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Kamato D, et al. Cell Signal. 2013 Oct;25(10):2017-24. doi: 10.1016/j.cellsig.2013.06.001. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770288 Review. - Smad regulation in TGF-beta signal transduction.
Moustakas A, Souchelnytskyi S, Heldin CH. Moustakas A, et al. J Cell Sci. 2001 Dec;114(Pt 24):4359-69. doi: 10.1242/jcs.114.24.4359. J Cell Sci. 2001. PMID: 11792802 Review.
Cited by
- Smad linker region phosphorylation in the regulation of extracellular matrix synthesis.
Burch ML, Zheng W, Little PJ. Burch ML, et al. Cell Mol Life Sci. 2011 Jan;68(1):97-107. doi: 10.1007/s00018-010-0514-4. Epub 2010 Sep 4. Cell Mol Life Sci. 2011. PMID: 20820849 Free PMC article. Review. - TGF-beta signal transduction in chronic kidney disease.
Schnaper HW, Jandeska S, Runyan CE, Hubchak SC, Basu RK, Curley JF, Smith RD, Hayashida T. Schnaper HW, et al. Front Biosci (Landmark Ed). 2009 Jan 1;14(7):2448-65. doi: 10.2741/3389. Front Biosci (Landmark Ed). 2009. PMID: 19273211 Free PMC article. Review. - CaMKII inhibition due to TRIC-B loss-of-function dysregulates SMAD signaling in osteogenesis imperfecta.
Besio R, Contento BM, Garibaldi N, Filibian M, Sonntag S, Shmerling D, Tonelli F, Biggiogera M, Brini M, Salmaso A, Jovanovic M, Marini JC, Rossi A, Forlino A. Besio R, et al. Matrix Biol. 2023 Jun;120:43-59. doi: 10.1016/j.matbio.2023.05.002. Epub 2023 May 11. Matrix Biol. 2023. PMID: 37178987 Free PMC article. - Repurposed drug screen identifies cardiac glycosides as inhibitors of TGF-β-induced cancer-associated fibroblast differentiation.
Coleman DT, Gray AL, Stephens CA, Scott ML, Cardelli JA. Coleman DT, et al. Oncotarget. 2016 May 31;7(22):32200-9. doi: 10.18632/oncotarget.8609. Oncotarget. 2016. PMID: 27058757 Free PMC article. - Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells.
Zhang P, Cai Y, Soofi A, Dressler GR. Zhang P, et al. J Biol Chem. 2012 Jun 15;287(25):21290-302. doi: 10.1074/jbc.M112.357202. Epub 2012 May 3. J Biol Chem. 2012. PMID: 22556418 Free PMC article.
References
- Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana J L. TGFβR1 phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. - PubMed
- Alevizopoulos A, Dusserre Y, Ruegg U, Mermod N. Regulation of the transforming growth factor β-responsive transcription factor CTF-1 by calcineurin and calcium/calmodulin-dependent protein kinase IV. J Biol Chem. 1997;272:23597–23605. - PubMed
- Antoine M, Gaiddon C, Loeffler J P. Ca2+/calmodulin kinase types II and IV regulate c-fos transcription in the AtT20 corticotroph cell line. Mol Cell Endocrinol. 1996;120:1–8. - PubMed
- Ashcroft G S, Yang X, Glick A B, Weinstein M, Letterio J J, Mizel D E, Anzano M, Greenwell-Wild T, Wahl S M, Deng C, Roberts A B. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. - PubMed
- Braun A P, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous