Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin - PubMed (original) (raw)
Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin
J V Shah et al. Mol Biol Cell. 2000 Oct.
Free PMC article
Abstract
Neuronal cytoskeletal elements such as neurofilaments, F-actin, and microtubules are actively translocated by an as yet unidentified mechanism. This report describes a novel interaction between neurofilaments and microtubule motor proteins that mediates the translocation of neurofilaments along microtubules in vitro. Native neurofilaments purified from spinal cord are transported along microtubules at rates of 100-1000 nm/s to both plus and minus ends. This motion requires ATP and is partially inhibited by vanadate, consistent with the activity of neurofilament-bound molecular motors. Motility is in part mediated by the dynein/dynactin motor complex and several kinesin-like proteins. This reconstituted motile system suggests how slow net movement of cytoskeletal polymers may be achieved by alternating activities of fast microtubule motors.
Figures
Figure 1
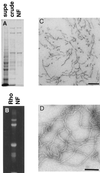
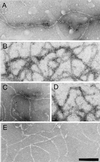
NFs purified from bovine spinal cord have a filamentous structure. (A) SDS-PAGE analysis of a subset of the fractions from the NF purification shows the progressive enrichment of NF triplet protein in the preparation. Supe refers to the supernatant remaining after centrifugation of crude NF, which was further purified over a sucrose cushion to give NF (see MATERIALS AND METHODS). (B) Fluorescent exposure of rhodamine-labeled NF fraction after SDS-PAGE analysis. (C) Electron micrograph of osmium tetroxide-stained sections of pelleted NF fraction. Bar, 200 nm. (D) Electron micrograph of negatively stained NFs from final NF fraction. Bar, 200 nm.
Figure 2
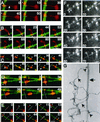
Neurofilaments translocate bidirectionally along microtubules. (A–E) Digitally colorized video sequences of fluorescently labeled NFs (red) translocating along a fluorescently labeled MT (green). White bars represent the position of the NFs at time zero. Arrowheads represent position of the NF on the MT at a particular point in time. In cases where NFs are filamentous the brightest intensity was taken as the position. Arrows indicate filamentous portions of NFs both along the MT (as in E), bound to glass (as in A) or waving in solution (as in C). The outstretched NFs in A (double arrow) and B demonstrate the filamentous nature of the preparation. (F) Uncolorized video sequence of NF translocation along MTs. The white line represents the initial position of the NF and the arrowhead and arrows represent the position of NF and filamentous portions thereof as described above. Bar (A–F), 5 μm. NF motility was carried out in the presence of 100 μM Mg2+-ATP. The plus and minus ends of polarity-marked MTs are indicated in those sequences that use polarity-marked MTs, as are the timings of the video sequences (in seconds). Video sequences corresponding to B, E, and F are available as supplementary information (2b&e.qt.mov and 2f.qt.mov). (G) An electron micrograph of NFs (arrows) and MTs (double arrow) prepared in the same manner as the motility assay demonstrates electron dense MT-NF contacts (arrowheads). Bar, 160 nm.
Figure 3
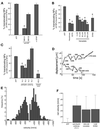
Analysis of neurofilament translocation. (A) Percentage of NFs translocating (of the total bound to MTs) in the presence of 100 μM Mg2+-ATP (baseline) and anti-IF antibodies. SMI31 and SMI32 recognize phosphorylated and nonphosphorylated NF epitopes, respectively. An anti-GFAP antibody is included as a control. Asterisk represents significant change in motility (p < 0.05) versus the GFAP control antibody. (B) NF motility is shown in the presence of ATP depletion by 2 U/ml hexokinase/5.5 mM glucose (Hexokinase) and Mg2+ depletion by 2 mM EDTA. Vanadate sensitivity for five concentrations (5, 10, 20, 70, 200 μM) and effect of 1 mM EHNA are also shown. Asterisks represent significant change in motility (p < 0.05) versus ATP alone. (C) NF transport after preincubation with the two anti-DynIC antibodies 74.1 and 70.1 and the corresponding Ig isotype controls, and a kinesin toxin (AS-2). Asterisks represent significant change in motility (p < 0.05) versus the appropriate control Ig (74.1 and 70.1) or ATP (AS-2). The control Ig values were not significantly different than ATP alone (p > 0.05). Heights of bars in A–C indicate the average (± SEM) of at least 10 independent video sequences comprising at least 150 NFs measured per condition. (D) Trajectories for two representative NFs demonstrate the bidirectional nature of the NF motility. Calculated velocities are shown for various segments of the trajectories. (E) Distribution of velocities of transported NFs in the presence of 100 μM Mg2+-ATP, n = 680 motile events from 90 different NFs. (F) Net velocities (mean ± SE) of NFs in the presence of dynein inhibitors (74.1 antibody, 200 μM vanadate, and 1 mM EHNA) and under control conditions (100 μM Mg2+-ATP), n > 40 motile events for each condition.
Figure 4
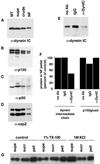
Dynein and dynactin complex members copurify with NFs. Fractions from the NF purification were probed with antibodies to dynein intermediate chain (A), p150glued (showing p150 and the neuronal p135 isoform) (B), p50 (dynamitin) (C), and capZ (α and β subunits) (D). Microtubules prepared from bovine brain were used as a positive control, and the NF fractions are those shown in Figure 1A. Approximately 20 μg of protein was loaded for supe, crude, and NF lanes and ∼11 μg for microtubule lanes. (E) The dynein–NF interaction was disrupted with an antibody to the intermediate chain of dynein (74.1) and the NFs pelleted and run on Western blots to determine the amount of dynein that remained bound to NFs. Controls without antibody and with an isotype IgG are shown for comparison. (F) Quantitation of dynein intermediate chain and p150glued immunoreactivity in NF pellets after incubation with the 74.1 dynein intermediate chain antibody, no antibody, or a control IgG. Values were normalized to NF content per lane and are shown as a percentage compared with no antibody controls. (G) NFs were subjected to 1% Triton X-100 (TX-100) or 1 M KCl extraction and pelleted over a sucrose cushion. Equal protein of fractions were separated by SDS-PAGE and probed for dynein intermediate chain immunoreactivity. Supernatant (supe) refers to the fraction above the sucrose cushion, sucrose (sucr) to the sucrose cushion, and pellet (pell) to the pelleted material. A control treatment with no detergent or KCl is also shown.
Figure 5
Dynein epitopes localize heterogeneously to NF contours. (A–D) Anti-dynein labeling of NFs shown by Immuno-EM by using anti-DynIC and secondary antibody conjugated to 8-nm gold beads. Anti-dynein antibody labels NFs above background levels and shows a heterogeneous pattern of labeling with areas of particularly dense antibody accumulation. (E) Control electron micrograph of NF treated with secondary antibody alone. Bar (A–E), 200 nm. (F) The number of gold beads was measured along 250-nm segments of NFs from one representative photomicrograph each of anti-dynein labeling and secondary antibody control. The total number of 250-nm segments is as follows: anti-dynein 119 and secondary antibody control 117. (G) The number of gold beads per micrometer of NF is shown for anti-dynein, anti-kinesin (SUK4), an IgG isotype control for the anti-dynein antibody, and a secondary antibody only control. The dynein-labeled NFs were separated into regions that were densely and sparsely labeled (see text). The density of labeling by NF-specific antibodies is included for comparison. The total length of NFs measured for each is as follows: anti-dynein 196.93 μm, anti-kinesin 92.96 μm, IgG control 85.86 μm, secondary only control 147.1 μm, and anti-NF 150.52 μm. The error bars represent the following number of photomicrographs: anti-dynein 5, anti-kinesin 5, IgG control 18, secondary only control 3, and NF 3.
Figure 6
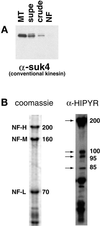
Kinesin-related proteins copurify with NFs. (A) Fractions from the NF purification were probed with the conventional kinesin heavy chain antibody SUK4. (B) The NF fraction was probed with the anti-HIPYR antibody. Arrows mark specific bands detected by the HIPYR antibody. The 200-kDa band does not comigrate with NF-H upon dephosphorylation (increased electrophoretic mobility) and is therefore distinct from NF-H (our unpublished results).
Figure 6
Kinesin-related proteins copurify with NFs. (A) Fractions from the NF purification were probed with the conventional kinesin heavy chain antibody SUK4. (B) The NF fraction was probed with the anti-HIPYR antibody. Arrows mark specific bands detected by the HIPYR antibody. The 200-kDa band does not comigrate with NF-H upon dephosphorylation (increased electrophoretic mobility) and is therefore distinct from NF-H (our unpublished results).
Similar articles
- The interaction of neurofilaments with the microtubule motor cytoplasmic dynein.
Wagner OI, Ascaño J, Tokito M, Leterrier JF, Janmey PA, Holzbaur EL. Wagner OI, et al. Mol Biol Cell. 2004 Nov;15(11):5092-100. doi: 10.1091/mbc.e04-05-0401. Epub 2004 Sep 1. Mol Biol Cell. 2004. PMID: 15342782 Free PMC article. - Intermediate filaments: vimentin moves in.
Clarke EJ, Allan V. Clarke EJ, et al. Curr Biol. 2002 Sep 3;12(17):R596-8. doi: 10.1016/s0960-9822(02)01102-8. Curr Biol. 2002. PMID: 12225682 Review. - A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules.
Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. Culver-Hanlon TL, et al. Nat Cell Biol. 2006 Mar;8(3):264-70. doi: 10.1038/ncb1370. Epub 2006 Feb 12. Nat Cell Biol. 2006. PMID: 16474384 - Cytoplasmic dynein and microtubule transport in the axon: the action connection.
Pfister KK. Pfister KK. Mol Neurobiol. 1999 Oct-Dec;20(2-3):81-91. doi: 10.1007/BF02742435. Mol Neurobiol. 1999. PMID: 10966115 Review. - Cytoplasmic dynein and dynactin as likely candidates for microtubule-dependent apical targeting of pancreatic zymogen granules.
Kraemer J, Schmitz F, Drenckhahn D. Kraemer J, et al. Eur J Cell Biol. 1999 Apr;78(4):265-77. doi: 10.1016/S0171-9335(99)80060-0. Eur J Cell Biol. 1999. PMID: 10350215
Cited by
- Neurofilament proteins in axonal regeneration and neurodegenerative diseases.
Wang H, Wu M, Zhan C, Ma E, Yang M, Yang X, Li Y. Wang H, et al. Neural Regen Res. 2012 Mar 15;7(8):620-6. doi: 10.3969/j.issn.1673-5374.2012.08.010. Neural Regen Res. 2012. PMID: 25745454 Free PMC article. Review. - Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport.
Rao MV, Garcia ML, Miyazaki Y, Gotow T, Yuan A, Mattina S, Ward CM, Calcutt NA, Uchiyama Y, Nixon RA, Cleveland DW. Rao MV, et al. J Cell Biol. 2002 Aug 19;158(4):681-93. doi: 10.1083/jcb.200202037. Epub 2002 Aug 19. J Cell Biol. 2002. PMID: 12186852 Free PMC article. - Axonal dynactin p150Glued transports caspase-8 to drive retrograde olfactory receptor neuron apoptosis.
Carson C, Saleh M, Fung FW, Nicholson DW, Roskams AJ. Carson C, et al. J Neurosci. 2005 Jun 29;25(26):6092-104. doi: 10.1523/JNEUROSCI.0707-05.2005. J Neurosci. 2005. PMID: 15987939 Free PMC article. - Neurofilaments switch between distinct mobile and stationary states during their transport along axons.
Trivedi N, Jung P, Brown A. Trivedi N, et al. J Neurosci. 2007 Jan 17;27(3):507-16. doi: 10.1523/JNEUROSCI.4227-06.2007. J Neurosci. 2007. PMID: 17234583 Free PMC article. - Loss of glial neurofascin155 delays developmental synapse elimination at the neuromuscular junction.
Roche SL, Sherman DL, Dissanayake K, Soucy G, Desmazieres A, Lamont DJ, Peles E, Julien JP, Wishart TM, Ribchester RR, Brophy PJ, Gillingwater TH. Roche SL, et al. J Neurosci. 2014 Sep 17;34(38):12904-18. doi: 10.1523/JNEUROSCI.1725-14.2014. J Neurosci. 2014. PMID: 25232125 Free PMC article.
References
- Cary RB, Klymkowsky MW, Evans RM, Domingo A, Dent JA, Backhus LE. Vimentin's tail interacts with actin-containing structures in vivo. J Cell Sci. 1994;107:1609–1622. - PubMed
- Cohn S, Saxton WM, Lye RJ, Scholey JM. Analyzing microtubule motors in real time. Methods Cell Biol. 1993;39:75–88. - PubMed
- Eyer J, McLean WG, Leterrier JF. Effect of a single dose of β,β′-iminodipropionitrile in vivo on the properties of neurofilaments in vitro: comparison with the effect of iminodipropionitrile added directly to neurofilaments in vitro. J Neurochem. 1989;52:1759–1765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources