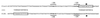Control of lactose transport, beta-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar - PubMed (original) (raw)
Control of lactose transport, beta-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar
P T van den Bogaard et al. J Bacteriol. 2000 Nov.
Abstract
Streptococcus thermophilus, unlike many other gram-positive bacteria, prefers lactose over glucose as the primary carbon and energy source. Moreover, lactose is not taken up by a phosphoenolpyruvate-dependent phosphotransferase system (PTS) but by the dedicated transporter LacS. In this paper we show that CcpA plays a crucial role in the fine-tuning of lactose transport, beta-galactosidase (LacZ) activity, and glycolysis to yield optimal glycolytic flux and growth rate. A catabolite-responsive element (cre) was identified in the promoter of the lacSZ operon, indicating a possible role for regulation by CcpA. Transcriptional analysis showed a sevenfold relief of repression in the absence of a functional CcpA when cells were grown on lactose. This CcpA-mediated repression of lacSZ transcription did not occur in wild-type cells during growth on galactose, taken up by the same LacS transport system. Lactose transport during fermentation was increased significantly in strains carrying a disrupted ccpA gene. Moreover, a ccpA disruption strain was found to release substantial amounts of glucose into the medium when grown on lactose. Transcriptional analysis of the ldh gene showed that expression was induced twofold during growth on lactose compared to glucose or galactose, in a CcpA-dependent manner. A reduced rate of glycolysis concomitant with an increased lactose transport rate could explain the observed expulsion of glucose in a ccpA disruption mutant. We propose that CcpA in S. thermophilus acts as a catabolic regulator during growth on the preferred non-PTS sugar lactose. In contrast to other bacteria, S. thermophilus possesses an overcapacity for lactose uptake that is repressed by CcpA to match the rate-limiting glycolytic flux.
Figures
FIG. 1
Primer extension analysis of the ccpA promoter. The transcriptional start site is indicated with an arrow. The −10 region in the coding strand is boxed. RNA was isolated from S. thermophilus CNRZ302 grown on glucose (G) or lactose (L), and primer extension products were run parallel to a sequence ladder (lanes A, C, G, and T) obtained with the same primer. Approximately 15 μg of RNA was used per primer extension reaction.
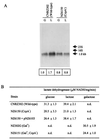
FIG. 2
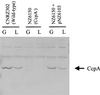
Western blot analysis of total protein extracts of S. thermophilus strains CNRZ302 (wild type), NZ6510 (CcpA−), and NZ6510 plus pNZ6103 grown on glucose (G) or lactose (L). Per sample, 10 μg of total protein was loaded, and CcpA proteins were detected using antibodies raised against B. megaterium CcpA. S. thermophilus CcpA was identified as a stained band of approximately 37 kDa. Next to the CcpA protein, several a-specific bands with a higher molecular weight were detected in every sample.
FIG. 3
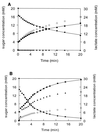
Small-scale fermentation of lactose of CNRZ302 (wild type) (A) and NZ6150 (ccpA disruption mutant) (B). Strains were grown to an OD600 of 1.0 in lactose-containing medium and resuspended in a 4% β-glycerophosphate buffer. Fermentation was started by addition of 20 mM lactose. Medium components were analyzed by HPLC. ●, lactose; ■, galactose; ▴, glucose; ◊, lactate.
FIG. 4
Alignment of the lacS and ldh promoter regions of S. thermophilus CNRZ302. The −35 and −10 boxes are in bold, and the determined transcriptional start sites are indicated by arrows. The putative cre sites are boxed and aligned with the consensus sequence.
FIG. 5
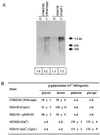
CcpA regulation of the S. thermophilus lacSZ operon. (A) Northern blot analysis of lacSZ expression of strains CNRZ302 (wild type) and NZ6510 (CcpA−) grown on glucose (G) or lactose (L). Below each lane, the relative amounts of the _lacSZ_-specific transcripts are given, which were obtained by phosphor image analysis of the Northern blot. These values were corrected for the total amount of RNA loaded; the lacSZ transcript amount of glucose-grown CNRZ302 was set at 1.0. (B) β-Galactosidase activities of the strains used in this study. Average values are presented of at least two independent experiments. n.d., not determined.
FIG. 6
CcpA regulation of the S. thermophilus ldh gene. (A) Northern blot analysis of ldh gene expression of strains CNRZ302 (wild type) and NZ6510 (CcpA−) grown on glucose (G) or lactose (L). Below each lane, the relative amounts of _ldh_-specific transcripts are given, which were obtained by phosphor image analysis of the Northern blot. These values were corrected for the total amount of RNA loaded; the ldh transcript amount of glucose-grown CNRZ302 was set at 1.0. (B) Lactate dehydrogenase activities of the strains used in this study. Average values are presented of at least two independent experiments. n.d., not determined.
Similar articles
- The extent of co-metabolism of glucose and galactose by Lactococcus lactis changes with the expression of the lacSZ operon from Streptococcus thermophilus.
Solem C, Koebmann B, Jensen PR. Solem C, et al. Biotechnol Appl Biochem. 2008 May;50(Pt 1):35-40. doi: 10.1042/BA20070157. Biotechnol Appl Biochem. 2008. PMID: 17822381 - Molecular characterization of CcpA and involvement of this protein in transcriptional regulation of lactate dehydrogenase and pyruvate formate-lyase in the ruminal bacterium Streptococcus bovis.
Asanuma N, Yoshii T, Hino T. Asanuma N, et al. Appl Environ Microbiol. 2004 Sep;70(9):5244-51. doi: 10.1128/AEM.70.9.5244-5251.2004. Appl Environ Microbiol. 2004. PMID: 15345406 Free PMC article. - Carbon catabolite repression by the catabolite control protein CcpA in Staphylococcus xylosus.
Jankovic I, Brückner R. Jankovic I, et al. J Mol Microbiol Biotechnol. 2002 May;4(3):309-14. J Mol Microbiol Biotechnol. 2002. PMID: 11931563 Review. - Carbon catabolite control of the metabolic network in Bacillus subtilis.
Fujita Y. Fujita Y. Biosci Biotechnol Biochem. 2009 Feb;73(2):245-59. doi: 10.1271/bbb.80479. Epub 2009 Feb 7. Biosci Biotechnol Biochem. 2009. PMID: 19202299 Review.
Cited by
- The doubly phosphorylated form of HPr, HPr(Ser~P)(His-P), is abundant in exponentially growing cells of Streptococcus thermophilus and phosphorylates the lactose transporter LacS as efficiently as HPr(His~P).
Cochu A, Roy D, Vaillancourt K, Lemay JD, Casabon I, Frenette M, Moineau S, Vadeboncoeur C. Cochu A, et al. Appl Environ Microbiol. 2005 Mar;71(3):1364-72. doi: 10.1128/AEM.71.3.1364-1372.2005. Appl Environ Microbiol. 2005. PMID: 15746339 Free PMC article. - UDP-N-acetylglucosamine 4-epimerase activity indicates the presence of N-acetylgalactosamine in exopolysaccharides of Streptococcus thermophilus strains.
Degeest B, Vaningelgem F, Laws AP, De Vuyst L. Degeest B, et al. Appl Environ Microbiol. 2001 Sep;67(9):3976-84. doi: 10.1128/AEM.67.9.3976-3984.2001. Appl Environ Microbiol. 2001. PMID: 11525994 Free PMC article. - Microbial Conversion of Protopanaxadiol-Type Ginsenosides by the Edible and Medicinal Mushroom Schizophyllum commune: A Green Biotransformation Strategy.
Liu Z, Li JX, Wang CZ, Zhang DL, Wen X, Ruan CC, Li Y, Yuan CS. Liu Z, et al. ACS Omega. 2019 Aug 1;4(8):13114-13123. doi: 10.1021/acsomega.9b01001. eCollection 2019 Aug 20. ACS Omega. 2019. PMID: 31460439 Free PMC article. - Complementary resource preferences spontaneously emerge in diauxic microbial communities.
Wang Z, Goyal A, Dubinkina V, George AB, Wang T, Fridman Y, Maslov S. Wang Z, et al. Nat Commun. 2021 Nov 18;12(1):6661. doi: 10.1038/s41467-021-27023-y. Nat Commun. 2021. PMID: 34795267 Free PMC article.
References
- Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
- Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. - PubMed
- de Vos W M, Simons G. Gene cloning and expression systems in lactococci. In Genetics and biotechnology of lactic acid bacteria. Gasson, M. G. and de Vos, W. M. (eds). Chapman and Hall, U.K. 1994. pp. 52–105.
- Egeter O, Brückner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. - PubMed
- Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol Microbiol. 1995;17:953–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous