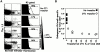T cells compete for access to antigen-bearing antigen-presenting cells - PubMed (original) (raw)
T cells compete for access to antigen-bearing antigen-presenting cells
R M Kedl et al. J Exp Med. 2000.
Abstract
These studies tested whether antigenic competition between T cells occurs. We generated CD8(+) T cell responses in H-2(b) mice against the dominant ovalbumin epitope SIINFEKL (ova8) and subdominant epitope KRVVFDKL, using either vaccinia virus expressing ovalbumin (VV-ova) or peptide-pulsed dendritic cells. CD8(+) T cell responses were visualized by major histocompatibility complex class I-peptide tetrameric molecules. Transfer of transgenic T cells with high affinity for ova8 (OT1 T cells) completely inhibited the response of host antigen-specific T cells to either antigen, demonstrating that T cells can directly compete with each other for response to antigen. OT1 cells also inhibited CD8(+) T cell responses to an unrelated peptide, SIYRYGGL, providing it was presented on the same dendritic cells as ova8. These inhibitions were not due to a more rapid clearance of virus or antigen-presenting cells (APCs) by the OT1 cells. Rather, the inhibition was caused by competition for antigen and antigen-bearing cells, since it could be overcome by the injection of large numbers of antigen-pulsed dendritic cells. These results imply that common properties of T cell responses, such as epitope dominance and secondary response affinity maturation, are the result of competitive interactions between antigen-bearing APC and T cell subsets.
Figures
Figure 1
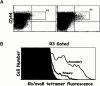
Tetramers can detect ovalbumin-specific T cells in VV-ova–immunized mice. B6 mice were immunized with 2 × 106 PFU VV-NP or VV-ova intravenously. 25 d later, some of the VV-ova–primed mice were reexposed to the same virus. 9 d after the primary infection or 5 d after the secondary infection, spleen and ovary cells were isolated from the animals, pooled, and stained with the Kb/ova8 tetramer and anti-CD8 as described in Materials and Methods. (A) Cells from mice infected once with VV-NP (left) or VV-ova (right). (B) Cells from mice undergoing primary or secondary responses to VV-ova. Background staining is from VV-NP–infected mice stained with the Kb/ova8 tetramer.
Figure 5
Competition of OT1 T cells with endogenous T cells is not antigen specific. B6.PL mice, with and without transfer of 5 × 105 OT1 T cells, were immunized with VV-ova intravenously. 9 d after the primary infection, spleen and ovary cells were isolated from the animals, pooled, and stained with the minor ovalbumin epitope Kb/KVVRDKL tetramer. Cells were analyzed as described in the legend to Fig. 2. Background staining was determined by irrelevant tetramer staining of experimental cells.
Figure 2
Transferred OT1 cells compete against the primary response of endogenous Kb/ova8-specific CD8+ T cells. B6.PL mice, with or without the transfer of 0.2–1 × 106 B6 OT1 transgenic T cells, were immunized with VV-ova as in the legend to Fig. 1. 9 d after infection, pooled ovary and spleen cells were stained with the Kb/ova8 tetramer as described in Materials and Methods. (A) Data were analyzed by gating on CD8+class II− events. The dot plots were additionally gated on all Thy1.2− events (R3 gate) to generate the histograms (right). The percentage given above the marker is the percentage of the total Thy1.2−CD8+ T cells staining positively for tetramer binding. Background (solid histogram) is from irrelevant tetramer staining of experimental cells. (B) Data pooled from two separate experiments demonstrating the inverse relationship between the expansion of the transferred cells and the expansion of the endogenous cells. The y-axis is the percentage of endogenous tetramer-staining cells out of all endogenous (Thy1.2−) cells. The x-axis is the percentage of transferred (Thy1.2+) cells out of both Thy1.2+ and Thy1.2− cells. The data shown are representative of six separate experiments.
Figure 3
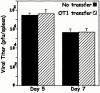
Transfer of OT1 cells does not cause accelerated clearance of VV-ova. B6.PL mice with or without transfer of 106 B6 OT1 transgenic T cells were immunized with VV-ova as in the legend to Fig. 1. On days 5 and 7 after infection, viral plaque assays were performed on pooled spleen and ovary tissue. The data shown are from triplicate titers of triplicate mice per time point. As a control, a small number of T cells were stained with tetramer from the transferred group of mice to insure that the transferred cells had indeed responded to the viral challenge (data not shown).
Figure 4
Transferred OT1 cells compete against the secondary response of endogenous Kb/ova8-specific CD8+ T cells and inhibit affinity maturation. 25 d after initial VV-ova immunization, B6.PL mice were transferred with 3 × 106 OT1 T cells and rechallenged with VV-ova. This response was compared with that of B6.PL mice given a secondary VV-ova immunization without OT1 T cell transfer. The data were analyzed as described in the legend to Fig. 2. The numbers above the markers in the histograms are the mean fluorescence intensity of the tetramer-staining cells. The data presented are representative of three separate experiments.
Figure 6
Peptide-pulsed DCs induce a vigorous CD8+ T cell response that can be inhibited by OT1 T cell transfer. Bone marrow was removed from the major leg bones of B6 mice, T and B cell depleted, and cultured with IL-4 and GM-CSF for 8 d. ova8 peptide was added to the cultures at 5 μg/ml for 2 h at 37°C. The resulting DCs were harvested and injected intravenously into B6 recipients at 106/mouse. On days 5, 7, and 9 after DC injection, the spleens were harvested and stained with Kb/ova8 tetramer. (A) Dot plots from the day 5 time point gated on live, CD8+class II− events. Companion stains showed the Kb/ova8 tetramer staining cells to also be predominantly blasts and CD25+ at only the day 5 time point, and L-selectinlo and CD44hi at all time points (data not shown). (B) Tetramer staining histograms of live, CD8+class II−CD44hi gated events of spleen cells from DC-challenged mice analyzed at the times indicated after initial DC challenge. (C) Mice immunized as in A with (+OT1) and without (No trans) the transfer of 5 × 105 OT1 T cells.
Figure 7
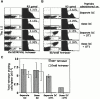
Non–antigen-specific competition of OT1 T cells is dependent on the copresentation of epitopes on a common APC. B6.PL mice with and without the transfer of 1–2 × 106 OT1 cells were challenged intravenously with antigen-pulsed DCs in two different ways: 1.5 × 106 0.5 μg/ml ova8-pulsed DCs plus 1.5 × 106 0.5 μg/ml SIYRYYGL-pulsed DCs (Separate DC); or 1.5 × 106 DCs pulsed with both peptides (Same DC). 5 d after DC challenge, the spleens were harvested and the cells were stained with either the Kb/SIYRYYGL tetramer (A) or the Kb/ova8 tetramer (B). The data for the dot plots were gated on live, CD8+class II− events, and the data for the histograms were further gated on Thy1.2− events (R3). The percentages given are the percentages of the total endogenous CD8+ T cell population. The total cell number was multiplied by the percent CD8+ in each sample, which was then multiplied by the percentage of tetramer-staining events in A and B to give the total number of tetramer-staining cells in each sample (C). The average and standard deviation were calculated from four mice per group.
Figure 8
Mice given OT1 T cells do not clear antigen-bearing DCs more rapidly than nontransferred mice. B6.PL mice with and without transfer of 2.5 × 105 OT1 T cells were challenged with increasing numbers of UBI-GFP bone marrow DCs pulsed with 5 ng/ml of ova8 peptide. DCs from the UBI-GFP mice were cultured as in the legend to Fig. 6. 5 d later, splenic DCs were isolated by collagenase treatment and assessed for the presence of GFP+ events. (A) GFP histograms of mice with and without transfer of OT1 cells. All GFP+ cells were also class II+ (not shown). (B) Percentage of GFP+ events of total spleen cells as shown in A, expressed as the average and standard deviation from three mice per group.
Figure 9
OT1 T cells compete with endogenous T cells for access to DCs. B6.PL mice with and without the transfer of 2.5 × 105 OT1 cells were challenged intravenously with B6 bone marrow DCs pulsed with 5 ng/ml of ova8 peptide. 5 d later, spleen cells were stained with tetramer as in the legend to Fig. 2. (A) Nontransferred B6.PL response to increasing numbers of DCs injected. The dot plots are gated on CD8+class II− events. The histograms were further gated on Thy1.2− (gate R3) events. (B) OT1-transferred B6.PL response to increasing numbers of DCs injected. Gating strategy as in A. (C) The average and standard deviation of endogenous T cell percentages from OT1-transferred and nontransferred mice, three mice per treatment.
Similar articles
- Peptide-pulsed splenic dendritic cells prime long-lasting CD8(+) T cell memory in the absence of cross-priming by host APC.
Livingstone AM, Kuhn M. Livingstone AM, et al. Eur J Immunol. 2002 Jan;32(1):281-90. doi: 10.1002/1521-4141(200201)32:1<281::AID-IMMU281>3.0.CO;2-P. Eur J Immunol. 2002. PMID: 11782019 - Enhanced antigen processing of flagellin fusion proteins promotes the antigen-specific CD8+ T cell response independently of TLR5 and MyD88.
Bates JT, Graff AH, Phipps JP, Grayson JM, Mizel SB. Bates JT, et al. J Immunol. 2011 Jun 1;186(11):6255-62. doi: 10.4049/jimmunol.1001855. Epub 2011 Apr 22. J Immunol. 2011. PMID: 21515787 Free PMC article. - TCR transgenic CD8+ T cells activated in the presence of TGFbeta express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection.
Kapp JA, Honjo K, Kapp LM, Xu Xy, Cozier A, Bucy RP. Kapp JA, et al. Int Immunol. 2006 Nov;18(11):1549-62. doi: 10.1093/intimm/dxl088. Epub 2006 Sep 11. Int Immunol. 2006. PMID: 16966495 - Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC.
Maecker HT, Umetsu DT, DeKruyff RH, Levy S. Maecker HT, et al. J Immunol. 1998 Dec 15;161(12):6532-6. J Immunol. 1998. PMID: 9862678 - Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.
Svensson M, Pfeifer J, Stockinger B, Wick MJ. Svensson M, et al. Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
Cited by
- Plasticity in programming of effector and memory CD8 T-cell formation.
Arens R, Schoenberger SP. Arens R, et al. Immunol Rev. 2010 May;235(1):190-205. doi: 10.1111/j.0105-2896.2010.00899.x. Immunol Rev. 2010. PMID: 20536564 Free PMC article. Review. - Unusual features of self-peptide/MHC binding by autoimmune T cell receptors.
Nicholson MJ, Hahn M, Wucherpfennig KW. Nicholson MJ, et al. Immunity. 2005 Oct;23(4):351-60. doi: 10.1016/j.immuni.2005.09.009. Immunity. 2005. PMID: 16226501 Free PMC article. Review. - Homeostatic competition among T cells revealed by conditional inactivation of the mouse Cd4 gene.
Wang Q, Strong J, Killeen N. Wang Q, et al. J Exp Med. 2001 Dec 17;194(12):1721-30. doi: 10.1084/jem.194.12.1721. J Exp Med. 2001. PMID: 11748274 Free PMC article. - Baculovirus-infected insect cells expressing peptide-MHC complexes elicit protective antitumor immunity.
Jordan KR, McMahan RH, Oh JZ, Pipeling MR, Pardoll DM, Kedl RM, Kappler JW, Slansky JE. Jordan KR, et al. J Immunol. 2008 Jan 1;180(1):188-97. doi: 10.4049/jimmunol.180.1.188. J Immunol. 2008. PMID: 18097019 Free PMC article. - The Function of Immunoproteasomes-An Immunologists' Perspective.
van den Eshof BL, Medfai L, Nolfi E, Wawrzyniuk M, Sijts AJAM. van den Eshof BL, et al. Cells. 2021 Nov 30;10(12):3360. doi: 10.3390/cells10123360. Cells. 2021. PMID: 34943869 Free PMC article. Review.
References
- Michaelis L. Untersuchungen uber Eiweissprazipitine. Dtsch. Med. Wschr. 1902;28:733.
- Michaelis L. Weitere Untersuchungen uber Eiweissprazipitine. Dtsch. Med. Wschr. 1904;30:1240.
- Liacopoulos P., Ben-Efraim S. Antigenic competition. Prog. Allergy. 1975;18:97–204. - PubMed
- Mitchison N.A. Specialization, tolerance, memory, competition, latency, and strife among T cells. Annu. Rev. Immunol. 1992;10:1–12. - PubMed
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI018785/AI/NIAID NIH HHS/United States
- AI22295/AI/NIAID NIH HHS/United States
- AI18785/AI/NIAID NIH HHS/United States
- R01 AI017134/AI/NIAID NIH HHS/United States
- R37 AI018785/AI/NIAID NIH HHS/United States
- R56 AI018785/AI/NIAID NIH HHS/United States
- P01 AI022295/AI/NIAID NIH HHS/United States
- R56 AI017134/AI/NIAID NIH HHS/United States
- AI17134/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous