Cross-talk in cell death signaling - PubMed (original) (raw)
Review
. 2000 Oct 16;192(8):F21-5.
Affiliations
- PMID: 11034612
- PMCID: PMC2195865
Review
Cross-talk in cell death signaling
S Roy et al. J Exp Med. 2000.
No abstract available
Figures
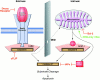
Figure 1
Extrinsic and intrinsic cell death signaling pathways. The majority of proteolytic cleavage events that manifest the apoptotic phenotype are mediated by ‘effector’ caspases, such as caspase-3 and caspase-7, which become fully activated when the large and small subunits that are harbored within the dormant proenzyme are liberated after endoproteolysis by upstream ‘initiator’ caspases, such as caspase-8 and caspase-9. The initiator caspases themselves are activated by autoproteolytic activation after facilitated oligomerization. In the extrinsic pathway, this occurs as a consequence of ligand binding to ‘death receptor’ complexes, which leads to the recruitment of procaspase-8 via the adapter molecule FADD/Mort1. This pathway is modulated by the availability of the molecular components (putative type I and type II cells differ in this regard) and dominant-negative regulators such as decoy receptors and cFLIP/usurpin. For the majority of cell death stimuli, the intrinsic death signal is communicated through the mitochondrion by an unknown mechanism, which leads to several changes in the organelle, including the release of polypeptidic agents dangereux, such as cytochrome c and second mitochondria-derived activator of caspases (SMAC)/Diablo. This pathway is highly dependent on the stoichiometry of anti- versus proapoptotic Bcl-2 family members. When enabled, caspase-9 activation occurs at the hands of the oligomerization mediator APAF-1, which requires cytochrome c for the appropriate conformation. SMAC/Diablo helps to cross another apoptosis checkpoint by sequestering inhibitors of apoptosis protein (IAPs), which would otherwise block the actions of downstream effector caspases even in the presence of proteolytic maturation. Controversy surrounds the degree to which cross-talk occurs between the extrinsic and intrinsic pathways in vivo. At a molecular level, this appears to occur via the proteolysis of BID, which normally serves an antiapoptotic role within the intrinsic pathway until it is truncated (to tBID) by caspase-8 (derived from the extrinsic pathway), whereupon it promotes activation of the intrinsic pathway. In principle, signals from the extrinsic pathway may require the assistance of the intrinsic pathway, for example, when the signal strength is weak or when the IAP barrier is high and the actions of SMAC/Diablo become necessary.
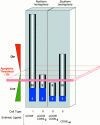
Figure 2
How apoptotic thresholding might influence experimental outcome. Many possible explanations may account for the differential sensitivity of cells from the ‘Northern hemisphere’ labs (e.g., Krammer/Peter) versus those from the ‘Southern hemisphere’ (e.g., Strasser) to CD95 stimulation (the Krammer/Peter labs are located in Germany and the Strasser lab is in Australia, hence the Northern and Southern hemisphere references.). Herein is illustrated one hypothesis. It assumes that most cell types have an apoptotic threshold that, when breached, results in apoptotic cell death. This apoptotic threshold (represented by the red plane) requires, say, 10 arbitrary apoptotic units (which could be, among other things, a critical mass of caspase catalytic activity). The ability of a cell to cross this threshold depends on the combined contributions of the extrinsic cell death pathway (gray) and the fraction of the intrinsic pathway (blue) that can be engaged through pathway cross-talk. Type I cells (first bar), in both the Northern and Southern hemispheres, harbor a very efficient DISC mechanism that alone can contribute the necessary apoptotic units to breach the threshold. Thus, regardless of the status of the intrinsic pathway, and even if it is completely blocked by the presence of Bcl-2, the type I cell can muster the necessary currency to engage the apoptotic machinery and die. Therefore, extrinsic and intrinsic pathways in type I cells appear independent (assuming intrinsic does not talk to extrinsic). In type II cells, DISC formation is weaker and the extrinsic pathway depends more on contributions from the intrinsic pathway. These cells behave differently in the Northern hemisphere versus the Southern hemisphere. In the Northern hemisphere, the combination of the extrinsic contribution and the fraction of the intrinsic pathway that it is able to engage is sufficient for apoptosis to proceed (second bar). Attenuation of the intrinsic component, by Bcl-2 overexpression for example, reduces the total number of apoptotic units to below the apoptotic threshold and the cells survive. The extrinsic pathway, therefore, appears to be modulated by Bcl-2. In the Southern hemisphere, the number of available apoptotic units that can be contributed by the intrinsic pathway may be lower. Thus, engagement of the extrinsic pathway with ligating CD95 (Fas/Apo1) antibody or trimerized ligand is not sufficient to mediate passage across the apoptotic threshold on its own (third bar). With a more efficient stimulus, mediated by multimerized CD95L, for example (fourth bar), a critical mass of the extrinsic pathway is enlisted to breach the apoptotic threshold, regardless of the contribution from the intrinsic pathway. Cell death in this scenario is therefore insensitive to Bcl-2 overexpression. The differences observed in the Northern and Southern hemispheres might therefore be explained by a combination of (a) the number of apoptotic units within the intrinsic pathway that can be recruited by cross-talk from the extrinsic pathway (lower in the South) and (b) the strength of the apoptotic stimulus (ligating antibodies and trimerized CD95L being equivalent but weaker than multimerized CD95L).
Comment on
- Fas ligand, Bcl-2, granulocyte colony-stimulating factor, and p38 mitogen-activated protein kinase: Regulators of distinct cell death and survival pathways in granulocytes.
Villunger A, O'Reilly LA, Holler N, Adams J, Strasser A. Villunger A, et al. J Exp Med. 2000 Sep 4;192(5):647-58. doi: 10.1084/jem.192.5.647. J Exp Med. 2000. PMID: 10974031 Free PMC article.
Similar articles
- Fas and Fas ligand in gut and liver.
Pinkoski MJ, Brunner T, Green DR, Lin T. Pinkoski MJ, et al. Am J Physiol Gastrointest Liver Physiol. 2000 Mar;278(3):G354-66. doi: 10.1152/ajpgi.2000.278.3.G354. Am J Physiol Gastrointest Liver Physiol. 2000. PMID: 10712254 Review. - TRPC6 channel activation promotes neonatal glomerular mesangial cell apoptosis via calcineurin/NFAT and FasL/Fas signaling pathways.
Soni H, Adebiyi A. Soni H, et al. Sci Rep. 2016 Jul 7;6:29041. doi: 10.1038/srep29041. Sci Rep. 2016. PMID: 27383564 Free PMC article. - Antagonism of CD95 signaling blocks butyrate induction of apoptosis in young adult mouse colonic cells.
Fan YY, Zhang J, Barhoumi R, Burghardt RC, Turner ND, Lupton JR, Chapkin RS. Fan YY, et al. Am J Physiol. 1999 Aug;277(2):C310-9. doi: 10.1152/ajpcell.1999.277.2.C310. Am J Physiol. 1999. PMID: 10444408 - Receptor cross-talk in angiogenesis: mapping environmental cues to cell phenotype using a stochastic, Boolean signaling network model.
Bauer AL, Jackson TL, Jiang Y, Rohlf T. Bauer AL, et al. J Theor Biol. 2010 Jun 7;264(3):838-46. doi: 10.1016/j.jtbi.2010.03.025. Epub 2010 Mar 20. J Theor Biol. 2010. PMID: 20307549 - Lipid and glycolipid mediators in CD95-induced apoptotic signaling.
Malisan F, Rippo MR, De Maria R, Testi R. Malisan F, et al. Results Probl Cell Differ. 1999;23:65-76. doi: 10.1007/978-3-540-69184-6_4. Results Probl Cell Differ. 1999. PMID: 9950029 Review. No abstract available.
Cited by
- Accelerated apoptosis contributes to aging-related hyperinflammation in endotoxemia.
Zhou M, Wu R, Dong W, Leong J, Wang P. Zhou M, et al. Int J Mol Med. 2010 Jun;25(6):929-35. doi: 10.3892/ijmm_00000424. Int J Mol Med. 2010. PMID: 20428798 Free PMC article. - Erianin inhibits the proliferation of T47D cells by inhibiting cell cycles, inducing apoptosis and suppressing migration.
Sun J, Fu X, Wang Y, Liu Y, Zhang Y, Hao T, Hu X. Sun J, et al. Am J Transl Res. 2016 Jul 15;8(7):3077-86. eCollection 2016. Am J Transl Res. 2016. PMID: 27508028 Free PMC article. - Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways.
Gaddy DF, Lyles DS. Gaddy DF, et al. J Virol. 2005 Apr;79(7):4170-9. doi: 10.1128/JVI.79.7.4170-4179.2005. J Virol. 2005. PMID: 15767418 Free PMC article. - Swine acute diarrhea syndrome coronavirus-induced apoptosis is caspase- and cyclophilin D- dependent.
Zhang J, Han Y, Shi H, Chen J, Zhang X, Wang X, Zhou L, Liu J, Zhang J, Ji Z, Jing Z, Ma J, Shi D, Feng L. Zhang J, et al. Emerg Microbes Infect. 2020 Feb 24;9(1):439-456. doi: 10.1080/22221751.2020.1722758. eCollection 2020. Emerg Microbes Infect. 2020. PMID: 32090691 Free PMC article. - Myxoma Virus Induces Ligand Independent Extrinsic Apoptosis in Human Myeloma Cells.
Bartee MY, Dunlap KM, Bartee E. Bartee MY, et al. Clin Lymphoma Myeloma Leuk. 2016 Apr;16(4):203-12. doi: 10.1016/j.clml.2015.12.005. Epub 2015 Dec 23. Clin Lymphoma Myeloma Leuk. 2016. PMID: 26803534 Free PMC article.
References
- Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. - PubMed
- Green D.R. Apoptotic pathwayspaper wraps stone blunts scissors. Cell. 2000;102:1–4. - PubMed
- Siegel R.M., Frederiksen J.K., Zacharias D.A., Chan F.K., Johnson M., Lynch D., Tsien R.Y., Lenardo M.J. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. - PubMed
- Chan F.K., Chun H.J., Zheng L., Siegel R.M., Bui K.L., Lenardo M.J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources