NO contributes to proliferative suppression in a murine model of filariasis - PubMed (original) (raw)
NO contributes to proliferative suppression in a murine model of filariasis
R A O'Connor et al. Infect Immun. 2000 Nov.
Abstract
Infection of BALB/c mice with microfilariae (mf) of Brugia pahangi leads to the suppression of antigen (Ag)-specific proliferative responses in the spleen. The proliferative defect is dependent on inducible nitric oxide synthase (iNOS) activity, since inhibition of iNOS with either L-N-monomethyl arginine (L-NMMA) or aminoguanidine reversed defective proliferation. Splenocytes from mf-infected animals produce high levels of gamma interferon (IFN-gamma) upon in vitro restimulation with Ag, and experiments in IFN-gamma receptor-deficient (IFN-gamma R(-/-)) mice demonstrated that signaling via the IFN-gamma R is essential in the induction of NO production and subsequent proliferative suppression. Restimulation of splenocytes from mf-infected animals with an extract of Acanthocheilonema viteae, a related filarial worm which lacks endosymbiotic bacteria, also resulted in NO production and proliferative suppression, demonstrating that lipopolysaccharide of bacterial origin is not essential to the induction of iNOS activity. These results extend previous observations that infection with different life cycle stages of Brugia leads to the development of differentially polarized immune responses and demonstrate one method by which these differences may exert their effects on the proliferative potential of cells from infected animals.
Figures
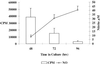
FIG. 1
Infection of BALB/c mice with mf results in proliferative suppression at 96 h. (A and B) Ag-stimulated proliferative responses of splenocytes from BALB/c mice infected i.v. with 105 mf, 50 L3 B. pahangi, or HBSS after 48 h (A) and 96 h (B) of in vitro restimulation with Ag (10 μg/ml) or medium only. Results are expressed as mean cpm incorporated in triplicate wells. (C) Ag-stimulated IFN-γ production after 48 h of culture. All values represent the mean and standard deviation for five animals per group.
FIG. 2
Proliferative suppression correlates with NO production by splenocytes from mf-infected animals. Nitrite production and proliferative responses of splenocytes from mf-infected BALB/c mice over a time course of in vitro restimulation with Ag (10 μg/ml) are shown. Data show the mean and standard deviation for five mice per group. Nitrite levels (right-hand axis) in culture display a strong negative correlation with proliferative responses (left-hand side) (r = −0.866, P = <0.001).
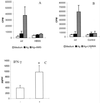
FIG. 3
Proliferative suppression can be reversed by inhibition of iNOS activity. (A and B) Proliferative responses of splenocytes from BALB/c mice given 105 B. pahangi mf or HBSS after 96 h of Ag-stimulated culture in the presence or absence of 500 μM AMG (A) or 250 μg of
l
-NMMA per ml (B). (C) IFN-γ production by splenocytes from mf-infected mice cultured in the presence or absence of AMG at 96 h of culture. All data represent the mean and standard deviation for five mice per group. ∗, significantly different from unsupplemented cultures (P < 0.05).
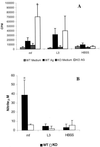
FIG. 4
NO production and proliferative suppression are dependent on IFN-γ. Proliferative responses (A) and nitrite production (B) of splenocytes from wild-type (129/Sv) and IFN-γR−/− mice given 105 mf or 50 L3 B. pahangi or HBSS i.v. after 96 h in vitro restimulation with Ag are shown. All data show the mean and standard deviation for five mice per group. ∗, significantly different from wild-type counterparts (P < 0.05).
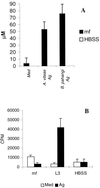
FIG. 5
Restimulation with A. viteae Ag induces NO production and proliferative suppression in splenocytes from mf-infected animals. (A) Nitrite production by splenocytes from B. pahangi mf-infected mice restimulated with 10 μg of A. viteae or B. pahangi Ag per ml, as shown. Cells from uninfected animals produced undetectable levels of nitrite in this experiment. (B) Proliferative responses of splenocytes from BALB/c mice given 105 mf, 50 L3 B. pahangi, or HBSS i.v. after 96 h of in vitro restimulation with A. viteae Ag (10 μg/ml) or medium only. All data show the mean and standard deviation for five mice per group. ∗, significantly different from wild-type counterparts (P < 0.05).
Similar articles
- Infection with Brugia microfilariae induces apoptosis of CD4(+) T lymphocytes: a mechanism of immune unresponsiveness in filariasis.
Jenson JS, O'Connor R, Osborne J, Devaney E. Jenson JS, et al. Eur J Immunol. 2002 Mar;32(3):858-67. doi: 10.1002/1521-4141(200203)32:3<858::AID-IMMU858>3.0.CO;2-E. Eur J Immunol. 2002. PMID: 11870630 - Interferon-gamma and nitric oxide production are not required for the immune-mediated clearance of Brugia malayi microfilariae in mice.
Gray CA, Lawrence RA. Gray CA, et al. Parasite Immunol. 2002 Jun;24(6):329-36. doi: 10.1046/j.1365-3024.2002.00464.x. Parasite Immunol. 2002. PMID: 12102718 - Nitric oxide limits the expansion of antigen-specific T cells in mice infected with the microfilariae of Brugia pahangi.
O'Connor RA, Devaney E. O'Connor RA, et al. Infect Immun. 2002 Nov;70(11):5997-6004. doi: 10.1128/IAI.70.11.5997-6004.2002. Infect Immun. 2002. PMID: 12379675 Free PMC article. - Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice.
Martins GA, Cardoso MA, Aliberti JC, Silva JS. Martins GA, et al. Immunol Lett. 1998 Sep;63(2):113-20. doi: 10.1016/s0165-2478(98)00066-2. Immunol Lett. 1998. PMID: 9761373 - Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria.
Ahvazi BC, Jacobs P, Stevenson MM. Ahvazi BC, et al. J Leukoc Biol. 1995 Jul;58(1):23-31. doi: 10.1002/jlb.58.1.23. J Leukoc Biol. 1995. PMID: 7542305
Cited by
- Differential cytokine and antibody responses to adult and larval stages of Onchocerca volvulus consistent with the development of concomitant immunity.
MacDonald AJ, Turaga PS, Harmon-Brown C, Tierney TJ, Bennett KE, McCarthy MC, Simonek SC, Enyong PA, Moukatte DW, Lustigman S. MacDonald AJ, et al. Infect Immun. 2002 Jun;70(6):2796-804. doi: 10.1128/IAI.70.6.2796-2804.2002. Infect Immun. 2002. PMID: 12010965 Free PMC article. - The effect of declining exposure on T cell-mediated immunity to Plasmodium falciparum - an epidemiological "natural experiment".
Bediako Y, Ngoi JM, Nyangweso G, Wambua J, Opiyo M, Nduati EW, Bejon P, Marsh K, Ndungu FM. Bediako Y, et al. BMC Med. 2016 Sep 22;14(1):143. doi: 10.1186/s12916-016-0683-6. BMC Med. 2016. PMID: 27660116 Free PMC article. - A Dirofilaria immitis polyprotein up-regulates nitric oxide production.
Tezuka H, Imai S, Tsukidate S, Fujita K. Tezuka H, et al. Infect Immun. 2002 Sep;70(9):5283-6. doi: 10.1128/IAI.70.9.5283-5286.2002. Infect Immun. 2002. PMID: 12183583 Free PMC article. - Resistance and susceptibility to filarial infection with Litomosoides sigmodontis are associated with early differences in parasite development and in localized immune reactions.
Babayan S, Ungeheuer MN, Martin C, Attout T, Belnoue E, Snounou G, Rénia L, Korenaga M, Bain O. Babayan S, et al. Infect Immun. 2003 Dec;71(12):6820-9. doi: 10.1128/IAI.71.12.6820-6829.2003. Infect Immun. 2003. PMID: 14638768 Free PMC article. - Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes.
Jenkins SJ, Allen JE. Jenkins SJ, et al. J Biomed Biotechnol. 2010;2010:262609. doi: 10.1155/2010/262609. Epub 2010 Jan 26. J Biomed Biotechnol. 2010. PMID: 20145705 Free PMC article. Review.
References
- Albina J E, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. - PubMed
- Allen J, Lawrence R A, Maizels R M. APC from mice harboring the filarial parasite, Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. - PubMed
- Allione A, Bernabei P, Bosticardo M, Fornii G, Novelli F. Nitric oxide suppresses human T lymphocyte proliferation through IFN-γ dependent and IFN-γ independent induction of apoptosis. J Immunol. 1999;163:4182–4191. - PubMed
- Corbett J A, Tilton R G, Chang K C, Hasan K S, Ido Y, Wang J I, Sweetland M A, Lancaster J R, Williamson J R, McDaniel M L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases