Detection and characterization of autoagglutination activity by Campylobacter jejuni - PubMed (original) (raw)
Detection and characterization of autoagglutination activity by Campylobacter jejuni
N Misawa et al. Infect Immun. 2000 Nov.
Free PMC article
Abstract
In several gram-negative bacterial pathogens, autoagglutination (AAG) activity is a marker for interaction with host cells and virulence. Campylobacter jejuni strains also show AAG, but this property varies considerably among strains. To examine the characteristics of C. jejuni AAG, we developed a quantitative in vitro assay. For strain 81-176, which shows high AAG, activity was optimal for cells grown for < or = 24 h, was independent of growth temperature, and was best measured for cells suspended in phosphate-buffered saline at 25 degrees C for 24 h. AAG activity was heat labile and was abolished by pronase or acid-glycine (pH 2.2) treatment but not by lipase, DNase, or sodium metaperiodate. Strain 4182 has low AAG activity, but extraction with water increased AAG, suggesting the loss of an inhibitor. Strain 6960 has weak AAG with no effect due to water extraction. Our study with clinical isolates suggests that C. jejuni strains may be grouped into three AAG phenotypes. A variant derived from strain 81116 that is flagellate but immotile showed the strong AAG exhibited by the parent strain, suggesting that motility per se is not necessary for the AAG activity. AAG correlated with both bacterial hydrophobicity and adherence to INT407 cells. Mutants which lack flagella (flaA, flaB, and flbA) or common cell surface antigen (peb1A) were constructed in strain 81-176 by natural transformation-mediated allelic exchange. Both AAG activity and bacterial hydrophobicity were abolished in the aflagellate mutants but not the peb1A mutant. In total, these findings indicate that C. jejuni AAG is highly associated with flagellar expression.
Figures
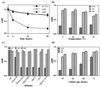
FIG. 1
Effect of assay conditions on the AAG activity of_C. jejuni_ strains 81-176, 4182, and 6960. (A) Duration of incubation time. (B) Incubation temperatures. (C) Diluent composition. (D) Age of the culture on TSAS plates. Each assay was conducted by suspending the bacterial cells in PBS (except for panel C), at 25°C (except for panel B) after a 24-h incubation (except for panel A), and the measuring _A_600. Bacterial cells that strongly agglutinate do not remain in the aqueous phase, and_A_600 diminishes.
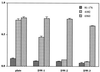
FIG. 2
Autoagglutination of cells of _C. jejuni_strains 81-176, 4182, and 6960 after sequential extraction with DW. The AAG assay was conducted in PBS at 25°C after 24-h incubation.
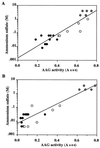
FIG. 3
Relationship between AAG activity and bacterial hydrophobicity, measured as minimum ammonium sulfate concentration permitting aggregation among 22 clinical C. jejuni isolates. Data are shown for cells before (A) and after (B) extraction with DW. For preextraction, r = 0.909 and P < 0.001, and for postextraction, r = 0.926 and_P_ < 0.001. The circles indicate three phenotypes: ●, substantially strong AAG and hydrophobicity (e.g., strain 81-176); ○, weak AAG and hydrophobicity, which increases after water extraction of cells (e.g., strain 4182);  , substantially weak AAG and hydrophobicity, not affected by water extraction (e.g., strain 6960).
, substantially weak AAG and hydrophobicity, not affected by water extraction (e.g., strain 6960).
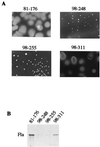
FIG. 4
Phenotypic characterization of the wild-type C. jejuni strain (81-176), the flaAflaB isogenic mutant (strain 98-248), the flbA isogenic mutant (strain 98-255), and the peb1A isogenic mutant (strain 98-311). (A) Motility analysis by semisolid agar assay. (B) Western blotting analysis of whole-cell extracts with rabbit polyclonal anti-flagellin antibody. Fla indicates the flagellin band present in strains 81-176 and 98-311 but absent from strains 98-248 and 98-255 as expected.
Similar articles
- Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis.
Golden NJ, Acheson DW. Golden NJ, et al. Infect Immun. 2002 Apr;70(4):1761-71. doi: 10.1128/IAI.70.4.1761-1771.2002. Infect Immun. 2002. PMID: 11895937 Free PMC article. - Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures.
Grant CC, Konkel ME, Cieplak W Jr, Tompkins LS. Grant CC, et al. Infect Immun. 1993 May;61(5):1764-71. doi: 10.1128/iai.61.5.1764-1771.1993. Infect Immun. 1993. PMID: 8478066 Free PMC article. - Effects of quorum sensing on flaA transcription and autoagglutination in Campylobacter jejuni.
Jeon B, Itoh K, Misawa N, Ryu S. Jeon B, et al. Microbiol Immunol. 2003;47(11):833-9. doi: 10.1111/j.1348-0421.2003.tb03449.x. Microbiol Immunol. 2003. PMID: 14638994 - Role of capsular polysaccharides and lipooligosaccharides in Campylobacter surface properties, autoagglutination, and attachment to abiotic surfaces.
Nguyen VT, Barlow RS, Fegan N, Turner MS, Dykes GA. Nguyen VT, et al. Foodborne Pathog Dis. 2013 Jun;10(6):506-13. doi: 10.1089/fpd.2012.1365. Epub 2013 Mar 28. Foodborne Pathog Dis. 2013. PMID: 23536985 - Campylobacter jejuni: components for adherence to and invasion of eukaryotic cells.
Lugert R, Gross U, Zautner AE. Lugert R, et al. Berl Munch Tierarztl Wochenschr. 2015 Mar-Apr;128(3-4):90-7. Berl Munch Tierarztl Wochenschr. 2015. PMID: 25876267 Review.
Cited by
- Baicalin Weakens the Virulence of Porcine Extraintestinal Pathogenic Escherichia coli by Inhibiting the LuxS/AI-2 Quorum-Sensing System.
Zong B, Xiao Y, Wang P, Liu W, Ren M, Li C, Fu S, Zhang Y, Qiu Y. Zong B, et al. Biomolecules. 2024 Apr 8;14(4):452. doi: 10.3390/biom14040452. Biomolecules. 2024. PMID: 38672469 Free PMC article. - Chimaeribacter arupi a new member of the Yersineacea family has the characteristics of a human pathogen.
Riediger M, Hoffmann K, Isberner R, Dreyer A, Tersteegen A, Marquardt P, Kaasch AJ, Zautner AE. Riediger M, et al. Front Cell Infect Microbiol. 2023 Oct 6;13:1277522. doi: 10.3389/fcimb.2023.1277522. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37868348 Free PMC article. - Evaluation of Bile Salts on the Survival and Modulation of Virulence of Aliarcobacter butzleri.
Mateus C, Maia CJ, Domingues F, Bücker R, Oleastro M, Ferreira S. Mateus C, et al. Antibiotics (Basel). 2023 Aug 30;12(9):1387. doi: 10.3390/antibiotics12091387. Antibiotics (Basel). 2023. PMID: 37760684 Free PMC article. - Direct interaction of small non-coding RNAs CjNC140 and CjNC110 optimizes expression of key pathogenic phenotypes of Campylobacter jejuni.
Ruddell B, Hassall A, Moss WN, Sahin O, Plummer PJ, Zhang Q, Kreuder AJ. Ruddell B, et al. mBio. 2023 Aug 31;14(4):e0083323. doi: 10.1128/mbio.00833-23. Epub 2023 Jul 6. mBio. 2023. PMID: 37409826 Free PMC article. - eDNA Provides a Scaffold for Autoaggregation of B. subtilis in Bacterioplankton Suspension.
Dogsa I, Kostanjšek R, Stopar D. Dogsa I, et al. Microorganisms. 2023 Jan 28;11(2):332. doi: 10.3390/microorganisms11020332. Microorganisms. 2023. PMID: 36838297 Free PMC article.
References
- Black R E, Levine M M, Clements M L, Hughes T P, Blaser M J. Experimental Campylobacter jejuniinfection in humans. J Infect Dis. 1988;157:472–479. - PubMed
- Butzler J P. Infection with campylobacters. In: Williams J D, editor. Modern topics in infection. London, United Kingdom: Heinemann; 1978. pp. 214–239.
- Chiang S L, Taylor R K, Koomey M, Mekakanos J J. Single amino acid substitutions in the N-terminus of Vibrio choleraeTcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources