Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease - PubMed (original) (raw)
Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease
A M Varnava et al. Heart. 2000 Nov.
Abstract
Objective: To make a quantitative assessment of the relation between disarray, fibrosis, and small vessel disease in hypertrophic cardiomyopathy.
Design: Detailed macroscopic and histological examination at 19 segments of the left and right ventricle and the left atrial free wall.
Patients: 72 patients with hypertrophic cardiomyopathy who had suffered sudden death or progression to end stage cardiac failure (resulting in death or heart transplantation).
Main outcome measures: The presence of scarring, atrial dilatation, and a mitral valve impact lesion were noted, and heart weight, wall thickness, per cent disarray, per cent fibrosis, and per cent small vessel disease quantitated for each heart.
Results: Within an individual heart the magnitude of hypertrophy correlated with the severity of fibrosis (p = 0.006) and disarray (p = 0.0002). Overall, however, total heart weight related weakly but significantly to fibrosis (r = 0.4, p = 0.0001) and small vessel disease (r = 0.3, p = 0.03), but not to disarray. Disarray was greater in hearts with mild left ventricular hypertrophy (maximum wall thickness < 20 mm) and preserved systolic function (60.9 (26)% v 43 (20.4)% respectively, p = 0.02) and hearts without a mitral valve impact lesion (26.3% v 18.9%, p = 0.04), but was uninfluenced by sex. Fibrosis was influenced by sex (7% in male patients and 4% in female, p = 0.04), but not by the presence of an impact lesion. No relation was found between disarray, fibrosis, and small vessel disease.
Conclusions: Myocyte disarray is probably a direct response to functional or structural abnormalities of the mutated sarcomeric protein, while fibrosis and small vessel disease are secondary phenomena unrelated to disarray, but modified by factors such as left ventricular mass, sex, and perhaps local autocrine factors.
Figures
Figure 1
Section showing focal distribution of myocyte disarray (to the left) adjacent to normal parallel alignment of myocytes (×16 objective).
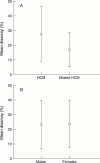
Figure 2
Disarray v disease status (A) and sex (B).
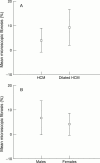
Figure 3
Microscopic fibrosis versus disease status (A) and sex (B).
Figure 4
Dysplastic small vessel with no surrounding microscopic fibrosis (×10 objective).
Figure 5
Pronounced fibrosis (sheets of collagen stained pink) with adjacent normal small vessel (×10 objective).
Similar articles
- Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy.
Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Varnava AM, et al. Am J Cardiol. 2001 Aug 1;88(3):275-9. doi: 10.1016/s0002-9149(01)01640-x. Am J Cardiol. 2001. PMID: 11472707 - Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease.
Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Varnava AM, et al. Circulation. 2001 Sep 18;104(12):1380-4. doi: 10.1161/hc3701.095952. Circulation. 2001. PMID: 11560853 - Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy.
Tanaka M, Fujiwara H, Onodera T, Wu DJ, Hamashima Y, Kawai C. Tanaka M, et al. Br Heart J. 1986 Jun;55(6):575-81. doi: 10.1136/hrt.55.6.575. Br Heart J. 1986. PMID: 3718796 Free PMC article. - Contractile Dysfunction in Sarcomeric Hypertrophic Cardiomyopathy.
MacIver DH, Clark AL. MacIver DH, et al. J Card Fail. 2016 Sep;22(9):731-7. doi: 10.1016/j.cardfail.2016.03.020. Epub 2016 May 16. J Card Fail. 2016. PMID: 27090746 Review. - [Myocardial interstitial fibrosis and diastolic dysfunction in hypertrophic cardiomyopathy].
Lombardi R, Betocchi S, Cacace A, Losi MA, Chiariello M. Lombardi R, et al. Ital Heart J Suppl. 2003 Aug;4(8):645-50. Ital Heart J Suppl. 2003. PMID: 14655460 Review. Italian.
Cited by
- A guide for assessment of myocardial stiffness in health and disease.
Villalobos Lizardi JC, Baranger J, Nguyen MB, Asnacios A, Malik A, Lumens J, Mertens L, Friedberg MK, Simmons CA, Pernot M, Villemain O. Villalobos Lizardi JC, et al. Nat Cardiovasc Res. 2022 Jan;1(1):8-22. doi: 10.1038/s44161-021-00007-3. Epub 2022 Jan 12. Nat Cardiovasc Res. 2022. PMID: 39196108 Review. - Echocardiographic Strain Abnormalities Precede Left Ventricular Hypertrophy Development in Hypertrophic Cardiomyopathy Mutation Carriers.
Canciello G, Lombardi R, Borrelli F, Ordine L, Chen SN, Santoro C, Frisso G, di Napoli S, Polizzi R, Cristiano S, Esposito G, Losi MA. Canciello G, et al. Int J Mol Sci. 2024 Jul 25;25(15):8128. doi: 10.3390/ijms25158128. Int J Mol Sci. 2024. PMID: 39125703 Free PMC article. - Cellular models and therapeutic perspectives in hypertrophic cardiomyopathy.
Yigit G, Wollnik B. Yigit G, et al. Med Genet. 2021 Dec 3;33(3):235-243. doi: 10.1515/medgen-2021-2094. eCollection 2021 Sep. Med Genet. 2021. PMID: 38835701 Free PMC article. - Substrate mechanics unveil early structural and functional pathology in iPSC micro-tissue models of hypertrophic cardiomyopathy.
Guo J, Jiang H, Schuftan D, Moreno JD, Ramahdita G, Aryan L, Bhagavan D, Silva J, Huebsch N. Guo J, et al. iScience. 2024 May 10;27(6):109954. doi: 10.1016/j.isci.2024.109954. eCollection 2024 Jun 21. iScience. 2024. PMID: 38827401 Free PMC article. - Effects of ranolazine on the arrhythmic substrate in hypertrophic cardiomyopathy.
Coleman JA, Doste R, Beltrami M, Argirò A, Coppini R, Olivotto I, Raman B, Bueno-Orovio A. Coleman JA, et al. Front Pharmacol. 2024 Apr 10;15:1379236. doi: 10.3389/fphar.2024.1379236. eCollection 2024. Front Pharmacol. 2024. PMID: 38659580 Free PMC article.
References
- Circulation. 1974 Sep;50(3):436-46 - PubMed
- Br Heart J. 1958 Jan;20(1):1-8 - PubMed
- Am Heart J. 1979 Jun;97(6):762-5 - PubMed
- Br Heart J. 1980 Oct;44(4):433-43 - PubMed
- Am Heart J. 1981 Jul;102(1):95-110 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources