Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt - PubMed (original) (raw)
Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt
F Scholle et al. J Virol. 2000 Nov.
Abstract
The Epstein-Barr virus LMP2A protein was expressed in a human keratinocyte cell line, HaCaT, and effects on epithelial cell growth were detected in organotypic raft cultures and in vivo in nude mice. Raft cultures derived from LMP2A-expressing cells were hyperproliferative, and epithelial differentiation was inhibited. The LMP2A-expressing HaCaT cells were able to grow anchorage independently and formed colonies in soft agar. HaCaT cells expressing LMP2A were highly tumorigenic and formed aggressive tumors in nude mice. The LMP2A tumors were poorly differentiated and highly proliferative, in contrast to occasional tumors that arose from parental HaCaT cells and vector control cells, which grew slowly and remained highly differentiated. Animals injected with LMP2A-expressing cells developed frequent metastases, which predominantly involved lymphoid organs. Involucrin, a marker of epithelial differentiation, and E-cadherin, involved in the maintenance of intercellular contact, were downregulated in LMP2A tumors. Whereas activation of the mitogen-activated protein kinase pathway was not observed, phosphatidylinositol-3-kinase (PI3-kinase)-dependent activation of the serine-threonine kinase Akt was detected in LMP2A-expressing cells and LMP2A tumors. Inhibition of this pathway blocked growth in soft agar. These data indicate that LMP2A greatly affects cell growth and differentiation pathways in epithelial cells, in part through activation of the PI3-kinase-Akt pathway.
Figures
FIG. 1
Characterization of HaCaT cell organotypic raft cultures. Hematoxylin-eosin stains of vector control (A) and LMP2A-expressing (B) HaCaT rafts are shown. LMP2A rafts are thickened with rounded cells containing large nuclei. Cells in vector control rafts are flattened, and enucleated cells are evident in the top layers of the culture. LMP2A expression was detected in the plasma membrane of cells in all layers of the epithelium using a rabbit HA antiserum and a biotin-streptavidin-peroxidase detection system (DAKO) (D). Background staining is evident in vector control rafts (C). Cell proliferation was determined by BrdU incorporation and staining with an anti-BrdU monoclonal antibody. Single BrdU-positive cells were observed in vector control rafts (E), while LMP2A-expressing rafts were highly proliferative (F). Localization of proliferating cells was not restricted to the basal cell layer. LMP2A blocks cell differentiation. The differentiation marker involucrin was present in the topmost layers of the epithelium of vector control rafts (G) but was not detected in LMP2A-expressing rafts (H).
FIG. 2
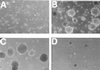
LMP2A-expressing HaCaT cells are anchorage independent. Vector control or LMP2A-expressing cells (6.7 × 104) were cultured in soft agar for 3 weeks. Vector control cells formed only small clumps of cells (A). Efficient colony formation was detected only in the HaCaT LMP2A cells (B). Growth in soft agar is PI3-kinase dependent. Colony formation was inhibited with a significant decrease in colony size in dishes treated with the PI3-kinase inhibitor LY294002 at 10 μM (D) but not in dimethyl sulfoxide-treated control dishes (C).
FIG. 3
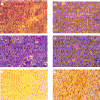
LMP2A expression induces tumorigenicity in nude mice. Hematoxylin-eosin stains of tumor sections of a parental tumor (A and C) and an LMP2A-induced tumor (B and D) are shown. Parental or LMP2A-expressing HaCaT cells (5 × 106) were injected subcutaneously into nude mice. The parental tumor, which appeared >8 weeks postinjection, was well differentiated, with a distinction between polarized and squamous cells. Abundant keratin whorls were detected. LMP2A tumors, which appeared after 1 to 2 weeks, were poorly differentiated. The cells contained large pleomorphic nuclei with distinct nucleoli. Blood vessels were abundant. No signs of differentiation were detected. (E and F) LMP2A-induced tumors were highly proliferative. Prior to sacrifice, animals were injected three times with BrdU at 50 mg/kg of body weight at 20-min intervals. Proliferating cells in the parental tumor (E) and the LMP2A tumor (F) were detected with an anti-BrdU antibody. Approximately 20 to 30% of the cells in LMP2A tumors stained positive for BrdU. Magnifications, A, B, E, and F, ×20; C and D, ×80.
FIG. 4
Immunoblot analysis of tumor lysates. (A) LMP2A expression detected with anti-HA polyclonal serum. Tumors expressed high levels of LMP2A (LIT1 to 3). LMP2A expression was not detected in a tumor arising form parental HaCaT cells (PT) or in a normal cervical lymph node (CLN). In contrast, a metastatic mesenteric lymph node (MLN) expressed high levels of LMP2A. (B) Involucrin expression detected with a mouse monoclonal antibody. Expression of involucrin was greatly decreased in LMP2A tumors, whereas fairly high levels were observed in both LMP2A and vector control cells in tissue culture. (C) LMP2A-expressing cells and tumors lose expression of E-cadherin. E-cadherin was detected by immunoblotting.
FIG. 5
LMP2A does not activate cell adhesion signaling or the MAPK pathway. Levels of focal adhesion kinase, Fak, expression in whole-cell extracts of the vector control, LMP2A-expressing cells, and tumors (LIT) are shown in the top panel. Phosphotyrosine (pTyr) immunoprecipitation (IP) from lysates of suspended (S) or adherent (A) fibronectin-stimulated cells and tumor lysates, followed by Fak and paxillin blotting, is shown in the second and third panels. Cell adhesion stimulates Fak and paxillin phosphorylation. Neither Fak nor paxillin was phosphorylated significantly in LMP2A tumors. MAPK activation was detected with an antibody recognizing the dually phosphorylated activated form. Cell adhesion to fibronectin stimulated ERK2, whereas neither ERK1 nor ERK2 was activated in LMP2A tumors (bottom panel).
FIG. 6
LMP2A expression activates Akt in a PI3-kinase-dependent fashion. (A) Immunoblot analysis with an antibody recognizing the activated form, phosphorylated on serine 473 (top). Akt activity is higher in LMP2A-expressing tissue culture cells and LMP2A tumors than in vector control cells. No activated (act.) Akt is detectable in a parental tumor (PT). PI3-kinase inhibition by wortmannin treatment for 30 min prior to harvesting of the cells leads to inhibition of Akt activity. Akt is expressed at similar levels in all of the cells and tissues examined (bottom). (B) Ribosomal S6 kinase is not activated by LMP2A expression. Tissue culture cell and LMP2A tumors (LIT) were analyzed by immunoblotting with an antibody that recognized the phosphorylated activated form of S6 kinase (top). S6 kinase levels are similar in all of the cells and tissues examined (bottom).
Similar articles
- Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway.
Fukuda M, Longnecker R. Fukuda M, et al. J Virol. 2007 Sep;81(17):9299-306. doi: 10.1128/JVI.00537-07. Epub 2007 Jun 20. J Virol. 2007. PMID: 17582000 Free PMC article. - Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells.
Morrison JA, Klingelhutz AJ, Raab-Traub N. Morrison JA, et al. J Virol. 2003 Nov;77(22):12276-84. doi: 10.1128/jvi.77.22.12276-12284.2003. J Virol. 2003. PMID: 14581564 Free PMC article. - Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway.
Portis T, Longnecker R. Portis T, et al. Oncogene. 2004 Nov 11;23(53):8619-28. doi: 10.1038/sj.onc.1207905. Oncogene. 2004. PMID: 15361852 - Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk- and Akt-dependent epithelial migration through αv-integrin membrane translocation.
Fotheringham JA, Coalson NE, Raab-Traub N. Fotheringham JA, et al. J Virol. 2012 Oct;86(19):10308-20. doi: 10.1128/JVI.00853-12. Epub 2012 Jul 25. J Virol. 2012. PMID: 22837212 Free PMC article. - Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction.
Miller CL, Lee JH, Kieff E, Burkhardt AL, Bolen JB, Longnecker R. Miller CL, et al. Infect Agents Dis. 1994 Apr-Jun;3(2-3):128-36. Infect Agents Dis. 1994. PMID: 7812651 Review.
Cited by
- Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9.
Lan YY, Hsiao JR, Chang KC, Chang JS, Chen CW, Lai HC, Wu SY, Yeh TH, Chang FH, Lin WH, Su IJ, Chang Y. Lan YY, et al. J Virol. 2012 Jun;86(12):6656-67. doi: 10.1128/JVI.00174-12. Epub 2012 Apr 18. J Virol. 2012. PMID: 22514348 Free PMC article. - Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma.
Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, Xiong D, Guan S, Guo BH, Mai HQ, Chen QY, Zhang X, Li MZ, Shao JY, Qian CN, Xia YF, Song LB, Zeng YX, Zeng MS. Kong QL, et al. PLoS Pathog. 2010 Jun 3;6(6):e1000940. doi: 10.1371/journal.ppat.1000940. PLoS Pathog. 2010. PMID: 20532215 Free PMC article. - Unique signaling properties of CTAR1 in LMP1-mediated transformation.
Mainou BA, Everly DN Jr, Raab-Traub N. Mainou BA, et al. J Virol. 2007 Sep;81(18):9680-92. doi: 10.1128/JVI.01001-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626074 Free PMC article. - The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility.
Allen MD, Young LS, Dawson CW. Allen MD, et al. J Virol. 2005 Feb;79(3):1789-802. doi: 10.1128/JVI.79.3.1789-1802.2005. J Virol. 2005. PMID: 15650203 Free PMC article. - Helicobacter pylori and Epstein-Barr virus infection in cell polarity alterations.
Baral B, Kandpal M, Ray A, Jana A, Yadav DS, Sachin K, Mishra A, Baig MS, Jha HC. Baral B, et al. Folia Microbiol (Praha). 2024 Feb;69(1):41-57. doi: 10.1007/s12223-023-01091-7. Epub 2023 Sep 6. Folia Microbiol (Praha). 2024. PMID: 37672163 Review.
References
- Alessi D R, Kozlowski M T, Weng Q P, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. - PubMed
- Assilineau D, Prunieras M. Reconstruction of ‘simplified’ skin: control of fabrication. Br J Dermatol. 1984;111:219–222. - PubMed
- Balendran A, Currie R, Armstrong C G, Avruch J, Alessi D R. Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J Biol Chem. 1999;274:37400–37406. - PubMed
- Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous