Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons - PubMed (original) (raw)
Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons
J T Kittler et al. J Neurosci. 2000.
Abstract
Type A GABA receptors (GABA(A)) mediate the majority of fast synaptic inhibition in the brain and are believed to be predominantly composed of alpha, beta, and gamma subunits. Although changes in cell surface GABA(A) receptor number have been postulated to be of importance in modulating inhibitory synaptic transmission, little is currently known on the mechanism used by neurons to modify surface receptor levels at inhibitory synapses. To address this issue, we have studied the cell surface expression and maintenance of GABA(A) receptors. Here we show that constitutive internalization of GABA(A) receptors in hippocampal neurons and recombinant receptors expressed in A293 cells is mediated by clathrin-dependent endocytosis. Furthermore, we identify an interaction between the GABA(A) receptor beta and gamma subunits with the adaptin complex AP2, which is critical for the recruitment of integral membrane proteins into clathrin-coated pits. GABA(A) receptors also colocalize with AP2 in cultured hippocampal neurons. Finally, blocking clathrin-dependant endocytosis with a peptide that disrupts the association between amphiphysin and dynamin causes a large sustained increase in the amplitude of miniature IPSCs in cultured hippocampal neurons. These results suggest that GABA(A) receptors cycle between the synaptic membrane and intracellular sites, and their association with AP2 followed by recruitment into clathrin-coated pits represents an important mechanism in the postsynaptic modulation of inhibitory synaptic transmission.
Figures
Fig. 1.
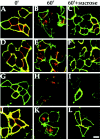
Clathrin-mediated endocytosis of recombinant GABAA receptors. A293 cells expressing α1β3γ2 (A–C, J–L), α1β3 (D–F), or γ2 (G–I) GABAA receptor subunits (all subunits 9E10-tagged) were prebound with anti-9E10 antibody (50 μg/ml) at 4°C for 30 min. Excess antibody was removed, and cells were incubated at 37°C for 60 min. Cells were then fixed, and cell surface receptors were detected in the absence of permeabilization with FITC-conjugated secondary antibody (green signal). The cells were then permeabilized with 0.05% Triton X-100. Internalized antibody was then measured using a secondary antibody conjugated with Texas Red (_red_signal). Significant internalization could be detected for all subunit combinations (B, E, H,K) after 60 min compared with 0 min (A, D, G,J). This internalization could be significantly inhibited by treatment with 350 m
m
sucrose to block clathrin-mediated endocytosis (C, F,I, L). Internalization in the presence of PKC activation by PDBu (K, L) was also blocked by 350 m
m
sucrose (L). Scale bar, 10 μ
m
.
Fig. 2.
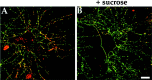
Clathrin-mediated endocytosis of GABAAreceptors in cultured hippocampal neurons. Neurons were labeled with anti-GABAA receptor β2/β3 subunit antibody incubated for 30 min at 37°C. Surface receptors were detected in the absence of permeabilization with FITC-conjugated secondary antibody followed by permeabilization and detection of internalized antibody with Texas Red-conjugated secondary antibody. Internalized antibody could be detected after 30 min (A). This internalization was blocked by treatment with 350 m
m
sucrose to block clathrin-mediated endocytosis (B). Scale bar, 10 μ
m
.
Fig. 3.
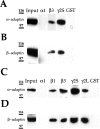
The intracellular domains of β1, β3, γ2S, and γ2L GABAA receptor subunits bind the adaptin α and β subunits of AP2. α1-GST, β3-GST, γ2S-GST, and GST were incubated with A293 cell extracts. After extensive washing, bound material was resolved by SDS-PAGE and analyzed by immunoblotting with an anti-α (A) or anti-β (B) adaptin antibody. α1-GST, β1-GST, β3-GST, γ2S-GST, γ2L-GST, or GST were incubated with brain extracts. After extensive washing, bound material was resolved by SDS-PAGE and analyzed by immunoblotting with an anti-α (C) or anti-β (D) adaptin antibody.
Fig. 4.
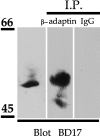
GABAA receptors immunoprecipitate with AP2 from brain. Detergent-solubilized brain extracts were immunoprecipitated with anti-β-adaptin or control IgG. Bound material was resolved by SDS-PAGE and analyzed by immunoblotting with an anti-GABAA receptor β2/β3 subunit antibody.
Fig. 5.
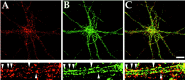
GABAA receptors colocalize with AP2 in clathrin-coated pits in cultured hippocampal neurons. Cultured hippocampal neurons (3 weeks old) were permeabilized and probed with a mouse anti-β-adaptin monoclonal antibody (A) and rabbit anti-GABAA receptor β1/β3 subunit polyclonal antibody (B). Antibodies were visualized with anti-rabbit FITC-conjugated and anti-mouse Texas Red-conjugated secondary antibodies. An enlargement of the same dendrite is shown in each panel. GABAA receptors, which colocalize with AP2, can be seen as_yellow_ clusters in the merged image in C(see arrowheads). Scale bar, 10 μ
m
.
Fig. 6.
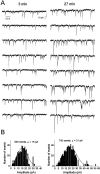
Blocking endocytosis increases the amplitude of GABAA-mediated mIPSCs in hippocampal neurons.A, Consecutive traces of mIPSCs selected 3 (left) and 27 (right) min after achieving whole-cell recording with pipette solution containing the endocytosis-blocking P4 peptide (50 μ
m
).B, Histograms showing amplitude distributions of a 4 min recording of mIPSCs starting at the time indicated in_A_.
Fig. 7.
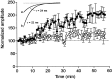
Time course of the effects of P4 endocytosis block on GABAA-mediated mIPSCs. Normalized mIPSC amplitude plotted against time after break-in with an internal solution containing either 50 μ
m
P4 (●) or 50 μ
m
SP control (○). The amplitude of the mIPSCs was calculated by averaging individual mIPSCs every 1 min recording. Each_point_ represents the mean ± SEM of four to five cells. The time on the _x_-axis represents the time after patch breakthrough. Inset, Single exponential decay of mIPSCs selected 3 (a) and 40 (b) min after break-in with the P4 peptide. No change in kinetics was detected during the enhancement of mIPSCs by the P4 peptide.
Similar articles
- Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization.
Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Man HY, et al. Neuron. 2000 Mar;25(3):649-62. doi: 10.1016/s0896-6273(00)81067-3. Neuron. 2000. PMID: 10774732 - Dynamin-dependent endocytosis of ionotropic glutamate receptors.
Carroll RC, Beattie EC, Xia H, Lüscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Carroll RC, et al. Proc Natl Acad Sci U S A. 1999 Nov 23;96(24):14112-7. doi: 10.1073/pnas.96.24.14112. Proc Natl Acad Sci U S A. 1999. PMID: 10570207 Free PMC article. - Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor.
Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Herring D, et al. J Biol Chem. 2003 Jun 27;278(26):24046-52. doi: 10.1074/jbc.M301420200. Epub 2003 Apr 21. J Biol Chem. 2003. PMID: 12707262 - The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition.
Vithlani M, Moss SJ. Vithlani M, et al. Biochem Soc Trans. 2009 Dec;37(Pt 6):1355-8. doi: 10.1042/BST0371355. Biochem Soc Trans. 2009. PMID: 19909275 Free PMC article. Review. - Intracellular trafficking of GABA(A) receptors.
Barnes EM Jr. Barnes EM Jr. Life Sci. 2000 Feb 11;66(12):1063-70. doi: 10.1016/s0024-3205(99)00469-5. Life Sci. 2000. PMID: 10737356 Review.
Cited by
- Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors.
Gonzalez C, Moss SJ, Olsen RW. Gonzalez C, et al. J Neurosci. 2012 Dec 5;32(49):17874-81. doi: 10.1523/JNEUROSCI.2535-12.2012. J Neurosci. 2012. PMID: 23223306 Free PMC article. - Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex.
Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Gu Z, et al. J Neurosci. 2005 May 18;25(20):4974-84. doi: 10.1523/JNEUROSCI.1086-05.2005. J Neurosci. 2005. PMID: 15901778 Free PMC article. - Endocytic Adaptor Proteins in Health and Disease: Lessons from Model Organisms and Human Mutations.
Azarnia Tehran D, López-Hernández T, Maritzen T. Azarnia Tehran D, et al. Cells. 2019 Oct 29;8(11):1345. doi: 10.3390/cells8111345. Cells. 2019. PMID: 31671891 Free PMC article. Review. - Autoimmune stiff person syndrome and related myelopathies: understanding of electrophysiological and immunological processes.
Rakocevic G, Floeter MK. Rakocevic G, et al. Muscle Nerve. 2012 May;45(5):623-34. doi: 10.1002/mus.23234. Muscle Nerve. 2012. PMID: 22499087 Free PMC article. Review. - Ubiquitin domain proteins in disease.
Madsen L, Schulze A, Seeger M, Hartmann-Petersen R. Madsen L, et al. BMC Biochem. 2007 Nov 22;8 Suppl 1(Suppl 1):S1. doi: 10.1186/1471-2091-8-S1-S1. BMC Biochem. 2007. PMID: 18047733 Free PMC article. Review.
References
- Barnes EM., Jr Use dependent regulation of GABAA receptors. Int Rev Neurobiol. 1996;39:54–70. - PubMed
- Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. The distribution, prevalence and drug binding profile of GABAA receptor subtypes differing in the β subunit variant. J Biol Chem. 1994;269:27100–27107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials