Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90 - PubMed (original) (raw)
Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90
A Chadli et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Heat shock protein (hsp)90 functions in a complex chaperoning pathway where its activity is modulated by ATP and by interaction with several co-chaperones. One co-chaperone, p23, binds selectively to the ATP-bound state of hsp90. However, the isolated ATP-binding domain of hsp90 does not bind p23. In an effort to identify the p23-binding domain, we have constructed a series of hsp90 deletion mutants fused with glutathione-S-transferase (GST). Full-length GST-hsp90 is able to bind p23, and also, to chaperone assembly of progesterone receptor complexes. Truncations from the C terminus of GST-hsp90 reveal a C-terminal boundary for the p23-binding domain at approximately residue 490. This fragment contains, in order, the ATP-binding domain, a highly charged region, and 203 residues beyond the charged region. p23 binding is unaffected by deletion of the charged region, indicating that two noncontiguous regions of hsp90 are involved in p23 binding. These regions are only effective when hsp90 is in a dimeric state as shown by loss of p23 binding upon removal of GST or as shown by use of FK506-binding protein12-hsp90 constructs that form dimers and bind p23 only in the presence of a bivalent drug. Thus, p23 binding requires an hsp90 dimer with close proximity between N-terminal regions of hsp90 and a conformation specified by ATP.
Figures
Figure 1
Mutations and structural domains of hsp90. (A) The location of two highly charged regions (A, 206–287 and B, 548–563), a potential leucine zipper region (Z, 378–429), and the conserved MEEVD at the C terminus of hsp90. Regions that bind ATP/geldanamycin (GA) (–14) or co-chaperones (–11) plus two domains that have been proposed for substrate binding (29, 30) are also indicated. (B) The hsp90 constructs used in this study are illustrated plus a summary of their activities for binding p23, Hop, and, ATP or for chaperoning PR as active (+), not active (−) or not tested (NT).
Figure 2
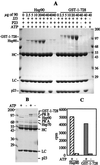
hsp90 and GST-1–728 have comparable p23-binding and PR assembly activities. (A) p23 (5 μg) was incubated with increasing concentrations of GST-1–728 or hsp90 in the presence of 2 mM ATP and the regeneration system as described in Materials and Methods. The proteins in complexes were resolved by SDS/PAGE. The position of M_r markers plus antibody heavy chain (HC) and light chain (LC) are indicated. The samples without hsp90 contained 40 μg of GST. (B and_C) Progesterone receptor complexes were assembled_in vitro_ in the presence of 5 mM ATP, 2 μg of Ydj 1, 20 μg of hsp70, 5 μg of Hop, 5 μg of p23, and either 20 μg of hsp90 or 26 μg of GST-1–728. Proteins in complex with PR were assessed by SDS/PAGE (B) and hormone-binding activity was measured (C).
Figure 3
Effects of hsp90 deletions on p23 binding. (A) GST-1–728 and GST-1–698 were compared for p23 binding in the presence or absence of 2 mM ATP. Background measurements were assessed by omitting p23 or the p23 antibody JJ3. (B) hsp90, GST-1–728, and GST-1–573 were assessed for p23 binding in the presence or absence of ATP, adenosine 5′-diphosphate, or geldanamycin (GA). (C) The GST-hsp90 constructs indicated were compared for p23 binding in the presence or absence of ATP. In all cases, the hsp90 constructs from p23 complexes were resolved by SDS/PAGE and stained with Coomassie Blue.
Figure 4
Binding of hsp90 mutants to p23. (A) GST-206–728 and GST-287–728 were assessed for p23 binding in the presence or absence of ATP. GST-1–728 was included as a positive control and the total protein assayed for each mutant is shown in lanes labeled (*) (B) p23 Binding is shown using hsp90 or the two full-length hsp90 mutants, R362/3A and L392S. The total protein assayed is shown in the last three lanes.
Figure 5
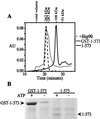
GST-dependent binding of 1–573 to p23. Fragment 1–573 was prepared by cleavage of GST (see Materials and Methods). (A) Size exclusion chromatography on Superdex 200 was used to compare the sizes of hsp90, GST-1–573, and 1–573. (B) The binding of p23 to GST-1–573 and to 1–573 is shown plus and minus ATP. Equivalent amounts of protein were used for the binding assay.
Figure 6
Dimer-dependent binding of p23. (A) SDS/PAGE analysis of purified 1–573 and FKBP-1–573 proteins. (B) Dimerization of FKBP-1–573 is dependent on the proportion of added bivalent drug. FKBP-1–573 was incubated for 1 h at 30° in the presence of the bivalent drug AP20187 (closely related to AP1903; ref. 38). drug/FKBP-1–573 ratios were varied between 0 and 2, and monomers (M) and dimers (D) were resolved by native PAGE. (C) p23 Binding correlates with drug-induced dimerization. Samples were prepared with FKBP-1–573 plus various ratios of AP20187 as in B and tested for p23-binding activity. Shown is the amount of FKBP-1–573 bound to p23 and resolved by SDS/PAGE and Western blotting with an antibody to hsp90.
Figure 7
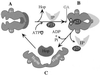
A model for the conversion of hsp90 through three conformational states. States A and B show differential interactions in the absence and presence of ATP. State C is an open nonfunctional conformation of hsp90.
Similar articles
- Polypeptide release by Hsp90 involves ATP hydrolysis and is enhanced by the co-chaperone p23.
Young JC, Hartl FU. Young JC, et al. EMBO J. 2000 Nov 1;19(21):5930-40. doi: 10.1093/emboj/19.21.5930. EMBO J. 2000. PMID: 11060043 Free PMC article. - Localization of sites of interaction between p23 and Hsp90 in solution.
Martinez-Yamout MA, Venkitakrishnan RP, Preece NE, Kroon G, Wright PE, Dyson HJ. Martinez-Yamout MA, et al. J Biol Chem. 2006 May 19;281(20):14457-64. doi: 10.1074/jbc.M601759200. Epub 2006 Mar 25. J Biol Chem. 2006. PMID: 16565516 - The influence of ATP and p23 on the conformation of hsp90.
Sullivan WP, Owen BA, Toft DO. Sullivan WP, et al. J Biol Chem. 2002 Nov 29;277(48):45942-8. doi: 10.1074/jbc.M207754200. Epub 2002 Sep 24. J Biol Chem. 2002. PMID: 12324468 - Evolutionary epitopes of Hsp90 and p23: implications for their interaction.
Zhu S, Tytgat J. Zhu S, et al. FASEB J. 2004 Jun;18(9):940-7. doi: 10.1096/fj.04-1570hyp. FASEB J. 2004. PMID: 15173105 Review. - p23, a simple protein with complex activities.
Felts SJ, Toft DO. Felts SJ, et al. Cell Stress Chaperones. 2003 Summer;8(2):108-13. doi: 10.1379/1466-1268(2003)008<0108:paspwc>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627195 Free PMC article. Review.
Cited by
- Hsp90: a specialized but essential protein-folding tool.
Young JC, Moarefi I, Hartl FU. Young JC, et al. J Cell Biol. 2001 Jul 23;154(2):267-73. doi: 10.1083/jcb.200104079. J Cell Biol. 2001. PMID: 11470816 Free PMC article. Review. - A novel method for detecting intramolecular coevolution: adding a further dimension to selective constraints analyses.
Fares MA, Travers SA. Fares MA, et al. Genetics. 2006 May;173(1):9-23. doi: 10.1534/genetics.105.053249. Epub 2006 Mar 17. Genetics. 2006. PMID: 16547113 Free PMC article. - Expression of a unique drug-resistant Hsp90 ortholog by the nematode Caenorhabditis elegans.
David CL, Smith HE, Raynes DA, Pulcini EJ, Whitesell L. David CL, et al. Cell Stress Chaperones. 2003 Spring;8(1):93-104. doi: 10.1379/1466-1268(2003)8<93:eoaudh>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 12820659 Free PMC article. - HSP90 at the hub of protein homeostasis: emerging mechanistic insights.
Taipale M, Jarosz DF, Lindquist S. Taipale M, et al. Nat Rev Mol Cell Biol. 2010 Jul;11(7):515-28. doi: 10.1038/nrm2918. Epub 2010 Jun 9. Nat Rev Mol Cell Biol. 2010. PMID: 20531426 Review. - Molecular imaging of the efficacy of heat shock protein 90 inhibitors in living subjects.
Chan CT, Paulmurugan R, Gheysens OS, Kim J, Chiosis G, Gambhir SS. Chan CT, et al. Cancer Res. 2008 Jan 1;68(1):216-26. doi: 10.1158/0008-5472.CAN-07-2268. Cancer Res. 2008. PMID: 18172314 Free PMC article.
References
- Caplan A J. Trends Cell Biol. 1999;9:262–268. - PubMed
- Buchner J. Trends Biochem Sci. 1999;24:136–141. - PubMed
- Mayer M P, Bukau B. Curr Biol. 1999;9:R322–R325. - PubMed
- Pratt W B. Proc Soc Exp Biol Med. 1998;217:420–434. - PubMed
- Smith D F. Mol Endocrinol. 1993;7:1418–1429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials