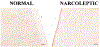Reduced number of hypocretin neurons in human narcolepsy - PubMed (original) (raw)
Reduced number of hypocretin neurons in human narcolepsy
T C Thannickal et al. Neuron. 2000 Sep.
Abstract
Murine and canine narcolepsy can be caused by mutations of the hypocretin (Hcrt) (orexin) precursor or Hcrt receptor genes. In contrast to these animal models, most human narcolepsy is not familial, is discordant in identical twins, and has not been linked to mutations of the Hcrt system. Thus, the cause of human narcolepsy remains unknown. Here we show that human narcoleptics have an 85%-95% reduction in the number of Hcrt neurons. Melanin-concentrating hormone (MCH) neurons, which are intermixed with Hcrt cells in the normal brain, are not reduced in number, indicating that cell loss is relatively specific for Hcrt neurons. The presence of gliosis in the hypocretin cell region is consistent with a degenerative process being the cause of the Hcrt cell loss in narcolepsy.
Figures
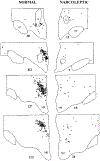
Figure 1.
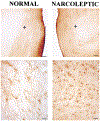
Distribution of Hypocretin-Labeled Somas in Normal and Narcoleptic Subjects Normal is subject CG, and narcoleptic is subject NA. Cell counts are listed below each section. On average, the narcoleptics had only 7% of the Hcrt cells seen in normals.
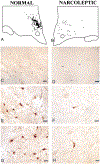
Figure 2.
Distribution of Cells in Perifornical and Dorsomedial Hypothalamic Regions of Normal and Narcoleptic Humans Plotted in Figure 1 All cells in figure are from the levels indicated in (A) and (B). (C and D) Low power photomicrographs (calibration [Cal.] = 100 μm) covering regions outlined in gray at the top shows many Hcrt somas and axons in the normal and few in the narcoleptic (arrows in narcoleptic indicate Hcrt somas verified as immunolabeled at higher magnification). Higher power photomicrographs (Cal. = 25μm) show Hcrt cells and axons in normal (E and G) and narcoleptic (F and H) subjects. Note the reduced density of axonal staining in the background of the narcoleptic as compared to that seen in the normal.
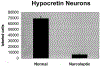
Figure 3.
The Number of Hcrt Cells Is Decreased in Narcoleptics
Figure 4.
Melanin-Concentrating Hormone Neurons in Normal and Narcoleptic Subjects Normal is subject CK, and narcoleptic is subject NA. Narcoleptics have normal numbers of MCH neurons despite the loss of 93% of Hcrt cells in the same region. Cal. = 250 μm.
Figure 5.
Glial Fibrillary Acidic Protein–Labeled Astrocytes in the Periventricular/Perifornical Region of a Normal and Narcoleptic Brain Low power photomicrograph is at the top, and a higher magnification of area marked with plus signs is at the bottom. Elevated GFAP staining in the hypothalamus of narcoleptics is consistent with a degenerative cause of cell loss. Top, Cal. = 500 μm; bottom, Cal. = 50 μm.
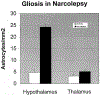
Figure 6.
Pattern of Gliosis in the Hypothalamus and Thalamus of Narcoleptics and Controls The number of GFAP labeled astrocytes is significantly increased in the hypothalamus of narcoleptics, the region containing Hcrt cells, but not in the thalamus.
Similar articles
- Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy.
Thannickal TC, Nienhuis R, Siegel JM. Thannickal TC, et al. Sleep. 2009 Aug;32(8):993-8. doi: 10.1093/sleep/32.8.993. Sleep. 2009. PMID: 19725250 Free PMC article. - Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy.
Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Thannickal TC, et al. Brain Pathol. 2003 Jul;13(3):340-51. doi: 10.1111/j.1750-3639.2003.tb00033.x. Brain Pathol. 2003. PMID: 12946023 Free PMC article. - Hypocretin (orexin) cell loss in Parkinson's disease.
Thannickal TC, Lai YY, Siegel JM. Thannickal TC, et al. Brain. 2007 Jun;130(Pt 6):1586-95. doi: 10.1093/brain/awm097. Epub 2007 May 9. Brain. 2007. PMID: 17491094 Free PMC article. - Hypocretin/orexin and narcolepsy: new basic and clinical insights.
Nishino S, Okuro M, Kotorii N, Anegawa E, Ishimaru Y, Matsumura M, Kanbayashi T. Nishino S, et al. Acta Physiol (Oxf). 2010 Mar;198(3):209-22. doi: 10.1111/j.1748-1716.2009.02012.x. Epub 2009 Jun 25. Acta Physiol (Oxf). 2010. PMID: 19555382 Free PMC article. Review. - Hypocretin (orexin): role in normal behavior and neuropathology.
Siegel JM. Siegel JM. Annu Rev Psychol. 2004;55:125-48. doi: 10.1146/annurev.psych.55.090902.141545. Annu Rev Psychol. 2004. PMID: 14744212 Free PMC article. Review.
Cited by
- Hypersomnia and depressive symptoms: methodological and clinical aspects.
Dauvilliers Y, Lopez R, Ohayon M, Bayard S. Dauvilliers Y, et al. BMC Med. 2013 Mar 21;11:78. doi: 10.1186/1741-7015-11-78. BMC Med. 2013. PMID: 23514569 Free PMC article. Review. - Role of the medial prefrontal cortex in cataplexy.
Oishi Y, Williams RH, Agostinelli L, Arrigoni E, Fuller PM, Mochizuki T, Saper CB, Scammell TE. Oishi Y, et al. J Neurosci. 2013 Jun 5;33(23):9743-51. doi: 10.1523/JNEUROSCI.0499-13.2013. J Neurosci. 2013. PMID: 23739971 Free PMC article. - Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: a proteomic approach.
van Steenoven I, Koel-Simmelink MJA, Vergouw LJM, Tijms BM, Piersma SR, Pham TV, Bridel C, Ferri GL, Cocco C, Noli B, Worley PF, Xiao MF, Xu D, Oeckl P, Otto M, van der Flier WM, de Jong FJ, Jimenez CR, Lemstra AW, Teunissen CE. van Steenoven I, et al. Mol Neurodegener. 2020 Jun 18;15(1):36. doi: 10.1186/s13024-020-00388-2. Mol Neurodegener. 2020. PMID: 32552841 Free PMC article. - Metabolic Regulation of Hypoxia-Inducible Factors in Hypothalamus.
Du D, Zhang Y, Zhu C, Chen H, Sun J. Du D, et al. Front Endocrinol (Lausanne). 2021 Mar 8;12:650284. doi: 10.3389/fendo.2021.650284. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33763034 Free PMC article. Review. - Evo-devo applied to sleep research: an approach whose time has come.
Brown RE. Brown RE. Sleep Adv. 2024 Jun 15;5(1):zpae040. doi: 10.1093/sleepadvances/zpae040. eCollection 2024. Sleep Adv. 2024. PMID: 39022590 Free PMC article. Review.
References
- Broberger C, De Lecea L, Sutcliffe JG, and Hokfelt T (1998). Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J. Comp. Neurol 402, 460–474. - PubMed
- Chemelli RM, Willie JT, Sinton C, Elmquist J, Scammell T, Lee C, Richardson J, Williams S, Xiong Y, Kisanuki Y, et al. (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451. - PubMed
- Erlich SS, and Itabashi HH (1986). Narcolepsy: a neuropathologic study. Sleep 9, 126–132. - PubMed
- Faraco JH, Rogers W, Overeem S, Nevsimalova S, Hungs M, Pedrazzoli M, Li R, and Fan J (2000). Mutation screening of hypocretin system genes in human narcoleptics. Sleep 23, A90–A91.
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL041370/HL/NHLBI NIH HHS/United States
- R01 NS014610/NS/NINDS NIH HHS/United States
- HL41370/HL/NHLBI NIH HHS/United States
- R01 MH064109/MH/NIMH NIH HHS/United States
- R37 NS014610/NS/NINDS NIH HHS/United States
- R01 HL041370/HL/NHLBI NIH HHS/United States
- NS14610/NS/NINDS NIH HHS/United States
- HL6029C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources