Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells - PubMed (original) (raw)
Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells
N Sevilla et al. J Exp Med. 2000.
Abstract
Among cells of the immune system, CD11c(+) and DEC-205(+) splenic dendritic cells primarily express the cellular receptor (alpha-dystroglycan [alpha-DG]) for lymphocytic choriomeningitis virus (LCMV). By selection, strains and variants of LCMV that bind alpha-DG with high affinity are associated with virus replication in the white pulp, show preferential replication in a majority of CD11c(+) and DEC-205(+) cells, cause immunosuppression, and establish a persistent infection. In contrast, viral strains and variants that bind with low affinity to alpha-DG are associated with viral replication in the red pulp, display minimal replication in CD11c(+) and DEC-205(+) cells, and generate a robust anti-LCMV cytotoxic T lymphocyte response that clears the virus infection. Differences in binding affinities can be mapped to a single amino acid change in the viral glycoprotein 1 ligand that binds to alpha-DG. These findings indicate that receptor-virus interaction on dendritic cells in vivo can be an essential step in the initiation of virus-induced immunosuppression and viral persistence.
Figures
Figure 1
VOPBA with purified α-DG and LCMV variants. Decreasing amounts (1, 0.1, and 0.01 μg) of purified α-DG were incubated with 107 PFU of each viral isolate and then with virus-specific antibody as described in Materials and Methods. The top panels show the VOPBA of CTL+P− LCMV isolates (ARM 53b, CD8-4, CD4-1, and TNFB2-1). The phenotype as well as the aa in position 260 of GP1 (F) is shown under each blot. The bottom panel shows the VOPBA of CTL−P+ LCMV isolates (Cl 13, PBL 7-1, PBL 36-4, PBL 50-1, PBL 67-3, and TNPBL4-2). The CTL P phenotype is indicated under the name of each isolate and the aa in position 260 of GP1 (L or I).
Figure 4
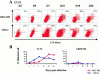
Replication of CTL−P+ and CTL+P− viruses in DCs. Four adult BALB/c ByJ mice were infected intravenously with 2 × 106 PFU of either ARM 53b or Cl 13. CD11c+ and DEC-205+ cells obtained from pooled spleens made into single splenocyte suspensions were labeled with specific antibodies for these cell subsets (see Materials and Methods). (A) Replication of Cl 13 in DCs. After intracellular staining with the monoclonal antibody specific to LCMV NP (113) and FACS®, dot plots were obtained that showed double-positive cells for 113-Alexa and DEC-205+ (top) or CD11c+ (bottom) cells. The numbers in each dot plot denote the percentage of 113-Alexa+ cells over the background in each cell population. (B) Curve plots show the replication of Cl 13 and ARM 53b in DEC-205+ and CD11c+ cells over a 20-d observation period.
Figure 2
Anatomic locations of viral nucleic acids within the spleen 3 d after infection with LCMV ARM 53b, Cl 13, and representative CTL−P+ (PBL 36-4, PBL 67-3, PBL 50-3, TNPBL4-2, and Mac 13-3) and CTL+P− variants (TNB2-1 and TNB4-1) of ARM 53b. In situ hybridization was performed using a digoxigenin-labeled riboprobe specific for LCMV NP. In spleen sections from BALB/c ByJ mice infected intravenously with 2 × 106 PFU of virus ARM 53b, TNB2-1, or TNB4-1, the nucleic acids localized predominantly in the red pulp (RP). Using identical conditions, the nucleic acids of CTL−P+ virus isolates localized within the white pulp (WP) or its marginal zone (MZ). All panels are shown at the same magnification. A higher power view is shown in the inset for ARM 53b– and Cl 13–infected mice. The arrows indicate viral nuleic acid detected in individual cells.
Figure 3
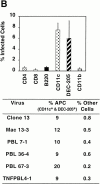
Comparative levels of infectivity in immune cells caused by CTL−P+ and CTL+P− viral variants. In each experiment, four adult BALB/c ByJ mice were injected intravenously with 2 × 106 PFU of virus, and 3 d after infection a pooled splenocyte suspension was prepared from each mouse (see Materials and Methods). Each cell population was labeled with cell-specific monoclonal antibodies to CD4, CD8, B220, CD11c, DEC-205, and CD11b. Subsequent intracellular staining using specific antibody to LCMV NP (antibody 113) was performed, followed by FACS®. The graph represents percentages of cells in each subset infected with ARM 53b (A) or Cl 13 (B). Summarized below are the percentages of infected antigen-presenting cells (CD11c, CD11b, B220, and DEC-205+ cells) or CD4 and CD8 T cells infected with these viruses. The term “other cells” refers to the sum of percentages for infected CD11b+, B220+, CD4+, and CD8+ T cells. (A) Infection of cell populations by CTL+P− viruses. (B) Infection of cell populations by CTL−P+ viruses.
Figure 3
Comparative levels of infectivity in immune cells caused by CTL−P+ and CTL+P− viral variants. In each experiment, four adult BALB/c ByJ mice were injected intravenously with 2 × 106 PFU of virus, and 3 d after infection a pooled splenocyte suspension was prepared from each mouse (see Materials and Methods). Each cell population was labeled with cell-specific monoclonal antibodies to CD4, CD8, B220, CD11c, DEC-205, and CD11b. Subsequent intracellular staining using specific antibody to LCMV NP (antibody 113) was performed, followed by FACS®. The graph represents percentages of cells in each subset infected with ARM 53b (A) or Cl 13 (B). Summarized below are the percentages of infected antigen-presenting cells (CD11c, CD11b, B220, and DEC-205+ cells) or CD4 and CD8 T cells infected with these viruses. The term “other cells” refers to the sum of percentages for infected CD11b+, B220+, CD4+, and CD8+ T cells. (A) Infection of cell populations by CTL+P− viruses. (B) Infection of cell populations by CTL−P+ viruses.
Figure 5
Comparison of binding affinities for the WE c54 and WE c2.2. isolates. (A) Biological properties of LCMV WE c54 and WE c2.2 as evaluated by the generation of an LCMV-specific CTL response at day 7 after infection and the ability or inability to clear the virus from the serum. CTL responses at day 7 are indicated as the percentage of 51Cr release from BALB/c ByJ (H-2d) targets at an effector/target ratio of 50:1, and the amount of infectious virus in serum is expressed as PFU per ml of plasma at day 30 after infection. The aa at position 153 of GP1 is indicated. (B) Comparison of the number of various splenic cell populations infected by LCMV WE c54 and WE c2.2. The results are shown as percentages of infected cells in each population as determined by FACS®. (A) VOPBA with decreasing amounts (1, 10−1, 10−2, and 10−3 μg) of purified α-DG blotted with 107 PFU of WE c54 or WE c2.2 virus detected with specific anti-GP LCMV antibodies as described in Materials and Methods. NIL, not detectable; ORF, open reading frame.
Figure 6
VOPBA with α-DG purified from fibroblasts (MC57 cells), total splenocytes, CD4+, CD8+, B220+, or CD11c+ cells incubated with LCMV Cl 13. α-DG purified from different numbers of cells (2 × 105 MC57 cells, 106 and 107 total splenocytes, and 2 × 106 CD11c+, CD4+, CD8+, and B220+ cells) was resolved in a 6% SDS-polyacrylamide gel. Incubation was with 108 PFU of Cl 13 and then with virus-specific antibody (see Materials and Methods). The arrow indicates the band corresponding to α-DG. The variability in molecular weights of α-DG from MC57 cells, total splenocytes, and CD11c+ cells likely reflects different glycosylation patterns.
Similar articles
- Infection of dendritic cells by lymphocytic choriomeningitis virus.
Sevilla N, Kunz S, McGavern D, Oldstone MB. Sevilla N, et al. Curr Top Microbiol Immunol. 2003;276:125-44. doi: 10.1007/978-3-662-06508-2_6. Curr Top Microbiol Immunol. 2003. PMID: 12797446 Free PMC article. Review. - Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics.
Smelt SC, Borrow P, Kunz S, Cao W, Tishon A, Lewicki H, Campbell KP, Oldstone MB. Smelt SC, et al. J Virol. 2001 Jan;75(1):448-57. doi: 10.1128/JVI.75.1.448-457.2001. J Virol. 2001. PMID: 11119613 Free PMC article. - Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan.
Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. Kunz S, et al. J Cell Biol. 2001 Oct 15;155(2):301-10. doi: 10.1083/jcb.200104103. Epub 2001 Oct 15. J Cell Biol. 2001. PMID: 11604425 Free PMC article. - Use of alternative receptors different than alpha-dystroglycan by selected isolates of lymphocytic choriomeningitis virus.
Kunz S, Sevilla N, Rojek JM, Oldstone MB. Kunz S, et al. Virology. 2004 Aug 1;325(2):432-45. doi: 10.1016/j.virol.2004.05.009. Virology. 2004. PMID: 15246281 - Viral escape from the neutralizing antibody response: the lymphocytic choriomeningitis virus model.
Ciurea A, Hunziker L, Zinkernagel RM, Hengartner H. Ciurea A, et al. Immunogenetics. 2001 Apr;53(3):185-9. doi: 10.1007/s002510100314. Immunogenetics. 2001. PMID: 11398962 Review.
Cited by
- The Multifaceted Roles of NK Cells in the Context of Murine Cytomegalovirus and Lymphocytic Choriomeningitis Virus Infections.
Hamdan TA. Hamdan TA. Immune Netw. 2024 Jun 27;24(4):e29. doi: 10.4110/in.2024.24.e29. eCollection 2024 Aug. Immune Netw. 2024. PMID: 39246620 Free PMC article. Review. - Pathogenic and Apathogenic Strains of Lymphocytic Choriomeningitis Virus Have Distinct Entry and Innate Immune Activation Pathways.
Johnson DM, Khakhum N, Wang M, Warner NL, Jokinen JD, Comer JE, Lukashevich IS. Johnson DM, et al. Viruses. 2024 Apr 19;16(4):635. doi: 10.3390/v16040635. Viruses. 2024. PMID: 38675975 Free PMC article. - The underlying mechanisms of arenaviral entry through matriglycan.
Katz M, Diskin R. Katz M, et al. Front Mol Biosci. 2024 Mar 7;11:1371551. doi: 10.3389/fmolb.2024.1371551. eCollection 2024. Front Mol Biosci. 2024. PMID: 38516183 Free PMC article. Review. - Mice with FVB-derived sequence on chromosome 17 succumb to disseminated virus infection due to aberrant NK cell and T cell responses.
Tibbs TN, Donoghue LJ, Buzzelli AA, Misumi I, DeMonia M, Ferris MT, Kelada SNP, Whitmire JK. Tibbs TN, et al. iScience. 2023 Oct 28;26(11):108348. doi: 10.1016/j.isci.2023.108348. eCollection 2023 Nov 17. iScience. 2023. PMID: 38026197 Free PMC article. - Does multiple sclerosis have a zoonotic origin? Correlations with lymphocytic choriomeningitis virus infection.
Hogeboom C. Hogeboom C. Front Immunol. 2023 Jun 16;14:1217176. doi: 10.3389/fimmu.2023.1217176. eCollection 2023. Front Immunol. 2023. PMID: 37398653 Free PMC article. No abstract available.
References
- de la Torre J.C., Oldstone M.B. Anatomy of viral persistencemechanisms of persistence and associated disease. Adv. Virus Res. 1996;46:311–343. - PubMed
- Oldstone M.B. Viruses can cause disease in the absence of morphological evidence of cell injuryimplication for uncovering new diseases in the future. J. Infect. Dis. 1989;159:384–389. - PubMed
- Fields, B.N., D.M. Knipe, and P.M. Howley. 1996. Persistance of viruses. In Virology. Lippincott-Raven, Philadelphia. 219–249.
- McChesney M.B., Oldstone M.B. Virus-induced immunosuppressioninfections with measles virus and human immunodeficiency virus. Adv. Immunol. 1989;45:335–380. - PubMed
- Doyle M.V., Oldstone M.B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J. Immunol. 1978;121:1262–1269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI45927/AI/NIAID NIH HHS/United States
- R01 AI045927/AI/NIAID NIH HHS/United States
- AI09484/AI/NIAID NIH HHS/United States
- R01 AI009484/AI/NIAID NIH HHS/United States
- P01 AG004342/AG/NIA NIH HHS/United States
- AG04342/AG/NIA NIH HHS/United States
- T32 AG000080/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials