The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells - PubMed (original) (raw)
The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells
F N Karanu et al. J Exp Med. 2000.
Abstract
The Notch ligand, Jagged-1, plays an essential role in tissue formation during embryonic development of primitive organisms. However, little is known regarding the role of Jagged-1 in the regulation of tissue-specific stem cells or its function in humans. Here, we show that uncommitted human hematopoietic cells and cells that comprise the putative blood stem cell microenvironment express Jagged-1 and the Notch receptors. Addition of a soluble form of human Jagged-1 to cultures of purified primitive human blood cells had modest effects in augmenting cytokine-induced proliferation of progenitors. However, intravenous transplantation of cultured cells into immunodeficient mice revealed that human (h)Jagged-1 induces the survival and expansion of human stem cells capable of pluripotent repopulating capacity. Our findings demonstrate that hJagged-1 represents a novel growth factor of human stem cells, thereby providing an opportunity for the clinical utility of Notch ligands in the expansion of primitive cells capable of hematopoietic reconstitution.
Figures
Figure 1
Expression of genes required for Notch signaling in purified human hematopoietic cells and their microenvironment. RT-PCR reactions were performed on cells using primers designed from known sequences available from the database. RT-PCR was performed on human fetal cDNA as a positive control while a housekeeping gene expressed at a single copy per cell, β-glucuronidase, was used to assess the quality and integrity of cDNA templates generated. (A) hNotch-1, hNotch-2, and hJagged-1 expression by cells of the hematopoietic environment of adult BM stroma and HUVECs. (B) Mature CD33+ myeloid cells, CD3+ T cells, CD19+ B cells, and primitive subfractions of CD34+CD38+Lin− and CD34+CD38−Lin− cells were isolated by flow cytometric sorting and reanalyzed to verify level of purification. Re-analysis revealed a >97% purity for all subfractions. (C) Expression of Notch signaling molecules hNotch-1, hNotch-2, and hJagged-1 in purified primitive Lin− cell subsets and mature cells from multiple lineages (CD33+ for myeloid, CD3+ for T cells, and CD19+ for B cells). PCR products were sequenced to verify specificity of gene amplification.
Figure 1
Expression of genes required for Notch signaling in purified human hematopoietic cells and their microenvironment. RT-PCR reactions were performed on cells using primers designed from known sequences available from the database. RT-PCR was performed on human fetal cDNA as a positive control while a housekeeping gene expressed at a single copy per cell, β-glucuronidase, was used to assess the quality and integrity of cDNA templates generated. (A) hNotch-1, hNotch-2, and hJagged-1 expression by cells of the hematopoietic environment of adult BM stroma and HUVECs. (B) Mature CD33+ myeloid cells, CD3+ T cells, CD19+ B cells, and primitive subfractions of CD34+CD38+Lin− and CD34+CD38−Lin− cells were isolated by flow cytometric sorting and reanalyzed to verify level of purification. Re-analysis revealed a >97% purity for all subfractions. (C) Expression of Notch signaling molecules hNotch-1, hNotch-2, and hJagged-1 in purified primitive Lin− cell subsets and mature cells from multiple lineages (CD33+ for myeloid, CD3+ for T cells, and CD19+ for B cells). PCR products were sequenced to verify specificity of gene amplification.
Figure 1
Expression of genes required for Notch signaling in purified human hematopoietic cells and their microenvironment. RT-PCR reactions were performed on cells using primers designed from known sequences available from the database. RT-PCR was performed on human fetal cDNA as a positive control while a housekeeping gene expressed at a single copy per cell, β-glucuronidase, was used to assess the quality and integrity of cDNA templates generated. (A) hNotch-1, hNotch-2, and hJagged-1 expression by cells of the hematopoietic environment of adult BM stroma and HUVECs. (B) Mature CD33+ myeloid cells, CD3+ T cells, CD19+ B cells, and primitive subfractions of CD34+CD38+Lin− and CD34+CD38−Lin− cells were isolated by flow cytometric sorting and reanalyzed to verify level of purification. Re-analysis revealed a >97% purity for all subfractions. (C) Expression of Notch signaling molecules hNotch-1, hNotch-2, and hJagged-1 in purified primitive Lin− cell subsets and mature cells from multiple lineages (CD33+ for myeloid, CD3+ for T cells, and CD19+ for B cells). PCR products were sequenced to verify specificity of gene amplification.
Figure 2
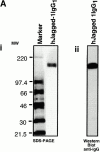
Characterization of soluble hJagged-1. (A) Analysis of Jagged-1 by Coomassie blue–stained SDS-PAGE (i) and Western blot analysis of purified hJagged-1 IgG1 chimera using secondary, goat anti–human IgG1 antibody (ii). MW is expressed in kilodaltons. (B) Specific binding of hJagged-1–FLAG chimera by human hematopoietic Lin− cells. Results are expressed as mean fluorescence intensity ± SEM compared with controls (n = 5). *Significant difference P < 0.05. * and ** are significantly different from each other, P < 0.01. hJagged-1–FLAG binding was competed by pretreatment of cells with hJagged-1 IgG1 chimera before treating with the FLAG-tagged ligand and by addition of 10 mM EDTA in staining buffer.
Figure 2
Characterization of soluble hJagged-1. (A) Analysis of Jagged-1 by Coomassie blue–stained SDS-PAGE (i) and Western blot analysis of purified hJagged-1 IgG1 chimera using secondary, goat anti–human IgG1 antibody (ii). MW is expressed in kilodaltons. (B) Specific binding of hJagged-1–FLAG chimera by human hematopoietic Lin− cells. Results are expressed as mean fluorescence intensity ± SEM compared with controls (n = 5). *Significant difference P < 0.05. * and ** are significantly different from each other, P < 0.01. hJagged-1–FLAG binding was competed by pretreatment of cells with hJagged-1 IgG1 chimera before treating with the FLAG-tagged ligand and by addition of 10 mM EDTA in staining buffer.
Figure 3
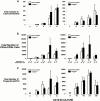
Functional in vitro analysis of primary human CD34+CD38−Lin− cells cultured in serum-free conditions containing hJagged-1. Highly purified CD34+CD38−Lin− cells were isolated (purity >99%, Fig. 1 B) and seeded in 96-well plates containing serum-free medium and hematopoietic cytokines in the presence of 10 μg/ml of soluble hJagged-1 or control recombinant IgG1. Cells were harvested at the indicated times, counted, phenotyped, and seeded into methylcellulose for progenitor cell assays. (A) The fold increase in total cell number relative to cells seeded on day 0. Cells from individual wells were counted, and the mean fold increase in absolute cell number was determined. Mean values ± SEM are shown. Viability of control treated and hJagged-1–treated cells was consistently >97%, indicating that increases in cell number and culture expansion are due to proliferation as opposed to differential survival (data not shown). (B) Changes in the total number of primitive CD34+CD38− cells in culture. The total number of CD34+CD38− cells was calculated using the total cell count from each well, and the frequencies of CD34+CD38− cells were determined by flow cytometry. The mean frequencies of CD34+CD38− cells are shown as a percentage below the treatment for each time point. (C) Effect of Jagged-1 on the total number of clonogenic progenitors. At the times indicated, an aliquot of cells was plated in methylcellulose culture. Total number of CFU per well was determined from the cell input number and number of colonies observed. Values shown are the mean ± SEM (n = 5). *Significant difference, P < 0.05.
Figure 4
Analysis of engraftment in NOD/SCID mice with CD34+CD38−Lin− cells cultured in the absence or presence hJagged-1. (A) Summary of levels of human cell engraftment in the BM of NOD/SCID mice transplanted with CD34+CD38−Lin− cells cultured in the absence (○) or presence (•) of Jagged-1 (10 μg/ml). Cells were cultured for the different periods indicated and transplanted by tail vein injection into NOD/SCID recipients. (i) Mice injected with 1,000–2,500 CD34+CD38−Lin− cells seeded on Day 0. (ii) Mice injected with 500–1,000 cells seeded on day 0. At days 12 and 15, horizontal bars indicate average level of human chimerism achieved in engrafted mice. (B) Representative Southern blot analysis of individual NOD/SCID mice transplanted with four equally divided aliquots of 2,500 CD34+CD38− cells seeded at day 0 and cultured under serum-free conditions for either 12 or 15 d in the presence or absence of hJagged-1. DNA was extracted from recipient murine BM 8 wk after the transplantation, separated on agarose gels, and hybridized with the human chromosome 17–specific α satellite probe. The level of engraftment was determined from the human/mouse DNA controls shown. (C) Multilineage differentiation of human repopulating cells in NOD/SCID mice after ex vivo culture with or without Jagged-1. Cells obtained from the BM of engrafted mice were stained with human specific mAbs and analyzed by flow cytometry. (i) Forward and side scatter properties were used to gate live cells in R1 for analysis. (ii) Isotype control for nonspecific IgG1 staining for PE and FITC fluorescence. Human cells (positive for panleukocyte marker CD45) were gated and analyzed for expression of the following human markers: (iii) pan-B cell markers for cells of the lymphoid lineage CD19 and CD20, (iv) myeloid markers CD15 and CD33, and (v) CD38 and primitive cell markers CD34. Results are shown for representative mice transplanted with control treated and Jagged-1–treated cultures.
Figure 4
Analysis of engraftment in NOD/SCID mice with CD34+CD38−Lin− cells cultured in the absence or presence hJagged-1. (A) Summary of levels of human cell engraftment in the BM of NOD/SCID mice transplanted with CD34+CD38−Lin− cells cultured in the absence (○) or presence (•) of Jagged-1 (10 μg/ml). Cells were cultured for the different periods indicated and transplanted by tail vein injection into NOD/SCID recipients. (i) Mice injected with 1,000–2,500 CD34+CD38−Lin− cells seeded on Day 0. (ii) Mice injected with 500–1,000 cells seeded on day 0. At days 12 and 15, horizontal bars indicate average level of human chimerism achieved in engrafted mice. (B) Representative Southern blot analysis of individual NOD/SCID mice transplanted with four equally divided aliquots of 2,500 CD34+CD38− cells seeded at day 0 and cultured under serum-free conditions for either 12 or 15 d in the presence or absence of hJagged-1. DNA was extracted from recipient murine BM 8 wk after the transplantation, separated on agarose gels, and hybridized with the human chromosome 17–specific α satellite probe. The level of engraftment was determined from the human/mouse DNA controls shown. (C) Multilineage differentiation of human repopulating cells in NOD/SCID mice after ex vivo culture with or without Jagged-1. Cells obtained from the BM of engrafted mice were stained with human specific mAbs and analyzed by flow cytometry. (i) Forward and side scatter properties were used to gate live cells in R1 for analysis. (ii) Isotype control for nonspecific IgG1 staining for PE and FITC fluorescence. Human cells (positive for panleukocyte marker CD45) were gated and analyzed for expression of the following human markers: (iii) pan-B cell markers for cells of the lymphoid lineage CD19 and CD20, (iv) myeloid markers CD15 and CD33, and (v) CD38 and primitive cell markers CD34. Results are shown for representative mice transplanted with control treated and Jagged-1–treated cultures.
Figure 4
Analysis of engraftment in NOD/SCID mice with CD34+CD38−Lin− cells cultured in the absence or presence hJagged-1. (A) Summary of levels of human cell engraftment in the BM of NOD/SCID mice transplanted with CD34+CD38−Lin− cells cultured in the absence (○) or presence (•) of Jagged-1 (10 μg/ml). Cells were cultured for the different periods indicated and transplanted by tail vein injection into NOD/SCID recipients. (i) Mice injected with 1,000–2,500 CD34+CD38−Lin− cells seeded on Day 0. (ii) Mice injected with 500–1,000 cells seeded on day 0. At days 12 and 15, horizontal bars indicate average level of human chimerism achieved in engrafted mice. (B) Representative Southern blot analysis of individual NOD/SCID mice transplanted with four equally divided aliquots of 2,500 CD34+CD38− cells seeded at day 0 and cultured under serum-free conditions for either 12 or 15 d in the presence or absence of hJagged-1. DNA was extracted from recipient murine BM 8 wk after the transplantation, separated on agarose gels, and hybridized with the human chromosome 17–specific α satellite probe. The level of engraftment was determined from the human/mouse DNA controls shown. (C) Multilineage differentiation of human repopulating cells in NOD/SCID mice after ex vivo culture with or without Jagged-1. Cells obtained from the BM of engrafted mice were stained with human specific mAbs and analyzed by flow cytometry. (i) Forward and side scatter properties were used to gate live cells in R1 for analysis. (ii) Isotype control for nonspecific IgG1 staining for PE and FITC fluorescence. Human cells (positive for panleukocyte marker CD45) were gated and analyzed for expression of the following human markers: (iii) pan-B cell markers for cells of the lymphoid lineage CD19 and CD20, (iv) myeloid markers CD15 and CD33, and (v) CD38 and primitive cell markers CD34. Results are shown for representative mice transplanted with control treated and Jagged-1–treated cultures.
Similar articles
- Soluble Jagged-1 is able to inhibit the function of its multivalent form to induce hematopoietic stem cell self-renewal in a surrogate in vitro assay.
Vas V, Szilágyi L, Pálóczi K, Uher F. Vas V, et al. J Leukoc Biol. 2004 Apr;75(4):714-20. doi: 10.1189/jlb.1003462. Epub 2004 Jan 23. J Leukoc Biol. 2004. PMID: 14742638 - Differential response of primitive human CD34- and CD34+ hematopoietic cells to the Notch ligand Jagged-1.
Karanu FN, Yuefei L, Gallacher L, Sakano S, Bhatia M. Karanu FN, et al. Leukemia. 2003 Jul;17(7):1366-74. doi: 10.1038/sj.leu.2402973. Leukemia. 2003. PMID: 12835726 - The soluble Notch ligand, Jagged-1, inhibits proliferation of CD34+ macrophage progenitors.
Masuya M, Katayama N, Hoshino N, Nishikawa H, Sakano S, Araki H, Mitani H, Suzuki H, Miyashita H, Kobayashi K, Nishii K, Minami N, Shiku H. Masuya M, et al. Int J Hematol. 2002 Apr;75(3):269-76. doi: 10.1007/BF02982040. Int J Hematol. 2002. PMID: 11999354 - Characterization of human hematopoietic cells with short-lived in vivo repopulating activity.
Eaves C, Glimm H, Eisterer W, Audet J, Maguer-Satta V, Piret J. Eaves C, et al. Ann N Y Acad Sci. 2001 Jun;938:63-70; discussion 70-1. doi: 10.1111/j.1749-6632.2001.tb03575.x. Ann N Y Acad Sci. 2001. PMID: 11458527 Review. - To be or not to be a pro-T?
Di Santo JP, Radtke F, Rodewald HR. Di Santo JP, et al. Curr Opin Immunol. 2000 Apr;12(2):159-65. doi: 10.1016/s0952-7915(99)00066-7. Curr Opin Immunol. 2000. PMID: 10712942 Review.
Cited by
- Inappropriate Notch activity and limited mesenchymal stem cell plasticity in the bone marrow of patients with myelodysplastic syndromes.
Varga G, Kiss J, Várkonyi J, Vas V, Farkas P, Pálóczi K, Uher F. Varga G, et al. Pathol Oncol Res. 2007;13(4):311-9. doi: 10.1007/BF02940310. Epub 2007 Dec 25. Pathol Oncol Res. 2007. PMID: 18158566 - Notch1 regulates progenitor cell proliferation and differentiation during mouse yolk sac hematopoiesis.
Cortegano I, Melgar-Rojas P, Luna-Zurita L, Siguero-Álvarez M, Marcos MA, Gaspar ML, de la Pompa JL. Cortegano I, et al. Cell Death Differ. 2014 Jul;21(7):1081-94. doi: 10.1038/cdd.2014.27. Epub 2014 Feb 28. Cell Death Differ. 2014. PMID: 24583642 Free PMC article. - Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells.
Zeng H, Yücel R, Kosan C, Klein-Hitpass L, Möröy T. Zeng H, et al. EMBO J. 2004 Oct 13;23(20):4116-25. doi: 10.1038/sj.emboj.7600419. Epub 2004 Sep 23. EMBO J. 2004. PMID: 15385956 Free PMC article. - Understanding intrinsic hematopoietic stem cell aging.
Mejia-Ramirez E, Florian MC. Mejia-Ramirez E, et al. Haematologica. 2020 Jan;105(1):22-37. doi: 10.3324/haematol.2018.211342. Epub 2019 Dec 5. Haematologica. 2020. PMID: 31806687 Free PMC article. Review.
References
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signalingcell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Watt F.M., Hogan B.L. Out of Edenstem cells and their niches. Science. 2000;287:1427–1430. - PubMed
- Weissman I.L. Translating stem and progenitor cell biology to the clinicbarriers and opportunities. Science. 2000;287:1442–1446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases