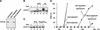Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts - PubMed (original) (raw)
Comparative Study
. 2000 Nov 1;14(21):2745-56.
doi: 10.1101/gad.842500.
Affiliations
- PMID: 11069891
- PMCID: PMC317027
- DOI: 10.1101/gad.842500
Comparative Study
Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts
Z Guo et al. Genes Dev. 2000.
Abstract
The checkpoint kinase Xchk1 becomes phosphorylated in Xenopus egg extracts in response to DNA replication blocks or UV-damaged DNA. Xchk1 is also required for the cell cycle delay that is induced by unreplicated or UV-damaged DNA. In this report, we have removed the Xenopus homolog of ATR (Xatr) from egg extracts by immunodepletion. In Xatr-depleted extracts, the checkpoint-associated phosphorylation of Xchk1 is abolished, and the cell cycle delay induced by replication blocks is strongly compromised. Xatr from egg extracts phosphorylated recombinant Xchk1 in vitro, but not a mutant form of Xchk1 (Xchk1-4AQ) containing nonphosphorylatable residues in its four conserved SQ/TQ motifs. Recombinant human ATR, but not a kinase-inactive mutant, phosphorylated the same sites in Xchk1. Furthermore, the Xchk1-4AQ mutant was found to be defective in mediating a checkpoint response in egg extracts. These findings suggest that Xchk1 is a functionally important target of Xatr during a checkpoint response to unreplicated or UV-damaged DNA.
Figures
Figure 1
Sequence of Xatr. (A) The COOH-terminal sequence. ATR homologs were aligned by using the PrettyPlot function of the GCG program. Identical residues are boxed. Sequences that were used to design the degenerate PCR primers are underlined. GenBank accession number for Xatr is AF223644. (B) Immunoblot analysis of Xatr and GST–Xatr. Endogenous Xatr in egg extracts (lane 1) and purified GST–Xatr (lane 2) were detected by immunoblotting with anti-Xatr antibodies against His6-Xatr(2351–2654).
Figure 1
Sequence of Xatr. (A) The COOH-terminal sequence. ATR homologs were aligned by using the PrettyPlot function of the GCG program. Identical residues are boxed. Sequences that were used to design the degenerate PCR primers are underlined. GenBank accession number for Xatr is AF223644. (B) Immunoblot analysis of Xatr and GST–Xatr. Endogenous Xatr in egg extracts (lane 1) and purified GST–Xatr (lane 2) were detected by immunoblotting with anti-Xatr antibodies against His6-Xatr(2351–2654).
Figure 2
Characterization of Xatr. (A) Xatr binds to DNA cellulose. Control cellulose (lane 2), single-stranded DNA cellulose (lanes 3–5), or double-stranded DNA cellulose (lanes 6,7) were incubated with 50 μL of cytosol in the absence or presence of aphidicolin (APH; 100 μg/mL; lanes 4,7) or protease inhibitors (PCL; 100 μg/mL each of pepstatin, chymostatin, and leupeptin; lane 5). Washed cellulose beads were boiled in 80 μL of gel loading buffer, half of which was subjected to immunoblot analysis for Xatr protein. Lane 1 depicts Xatr in the egg extract. (B) DNaseI digestion partially released Xatr and RPA70 from DNA cellulose. Single-stranded DNA cellulose (lanes 1,3) and control cellulose (lanes 2,4) that had been incubated with cytosol and washed were incubated with buffer alone (lanes 1,2) or DNaseI (lanes 3,4). The proteins that still bound to cellulose were separated by SDS-PAGE and transferred to a PVDF membrane. The Xatr and RPA70 proteins were detected by immunoblot. (C) DNA mediated the coimmunoprecipitation of Xatr and RPA70. Proteins associated with single-stranded DNA cellulose (lanes 1,2,5,6) or control cellulose (lanes 3,4) were released with either DNaseI treatment (lanes 1,2) or 0.5% NP-40 (lanes 3–6). The released proteins were incubated with either anti-Xatr antibodies or nonspecific IgG. The immunoprecipitates were subjected to immunoblot analysis with anti-Xatr (upper panel) and anti-RPA70 antibodies (lower panel). (D) Sensitivity of Xatr-associated kinase activity to caffeine. Kinase assays were performed by incubating the anti-Xatr immunoprecipitates (lanes 1–5) or control immunoprecipitates (lanes 6–10) with PHAS-I (1 μg) and 0–5 mM caffeine, as indicated. Proteins were separated by SDS-PAGE and visualized by silver staining. Phosphorylation of PHAS-I was detected by autoradiography. (E) Xatr released from DNA cellulose displays increased kinase activity. Xatr was immunoprecipitated from cytosol (lane 1) or from DNA cellulose-associated proteins that were released by DNaseI treatment (lane 2). Xatr-associated kinase activity was measured by using PHAS-I as the substrate. As a control, nonspecific IgG did not immunoprecipitate any kinase activity toward PHAS-I from either cytosol or DNA cellulose eluates (lanes 3,4). Xatr immunoprecipitated from both M13 DNA cellulose and pBluescript DNA cellulose eluates displayed similarly elevated kinase activity. Shown here is the result obtained with M13 DNA cellulose.
Figure 3
The effects of Xatr immunodepletion. (A) Immunodepletion of Xatr. Egg extracts depleted with affinity-purified anti-Xatr antibodies (made against His6-Xatr(2351–2654); lane 2) or nonspecific rabbit IgG (lane 3), along with untreated extract (lane 1), were analyzed for Xatr protein by immunoblotting. (B) Effects of Xatr depletion on the phosphorylation of Xchk1. Extracts depleted of Xatr (lanes 2,4,6) or mock-depleted extracts (lanes 1,3,5) containing sperm nuclei (2000 nuclei/μL; lanes 1,2), sperm nuclei (2000 nuclei/μL) and aphidicolin (100 μg/mL; lanes 3,4), or UV-damaged nuclei (2000 nuclei/μL; lanes 5,6) were incubated at 23°C for 100 min in the presence of 100 μg/mL of cycloheximide. Xchk1 present in the nuclear fraction was detected by immunoblot analysis. (C) Effects of Xatr depletion on the phosphorylation of Xcds1. Extracts depleted of Xatr (lanes 2,4) or mock-depleted extracts (lanes 1,3,5) containing no DNA (lane 1), M13 DNA (10 ng/μL; lanes 2,3), or poly(dT)40 (50 ng/μL; lanes 4,5) were incubated at 23°C for 100 min in the presence of 100 μg/mL of cycloheximide before analysis for Xcds1 protein by immunoblotting. (D) Immunodepletion of Xatr compromised the cell cycle delay induced by DNA replication blocks. Xatr-depleted egg extracts (open circles, filled circles) or mock-depleted extracts (open squares, filled squares) were activated with CaCl2 before the addition of sperm nuclei (500 nuclei/μL; open circles, open squares) or both sperm nuclei (1000 nuclei/μL) and aphidicolin (100 μg/mL) (filled circles, filled squares). The timing of nuclear envelope breakdown (NEB) was monitored by microscopy.
Figure 4
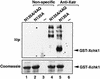
Phosphorylation of bacterially expressed, full-length GST-Xchk1 by Xatr in vitro. Xatr immunoprecipitated from eluates of DNA (pBluescript) cellulose was incubated with GST-Xchk1-N135A (lane 5), GST-Xchk1-N135A-4AQ (lane 4), or no substrate (lane 6) in the presence of 32P-ATP. The corresponding immunoprecipitates with nonspecific IgG were assayed in lanes 1–3. The proteins were separated by SDS-PAGE and visualized by Coomassie blue staining (bottom panel). Phosphorylated proteins were detected by autoradiography (top panel).
Figure 5
Xatr and human ATR phosphorylate Xchk1 at SQ/TQ sites. (A) The fusion proteins GST-Xchk1(347–374), GST-Xchk1(306–352), and the indicated SQ/TQ→AQ mutants of these proteins were tested as substrates in kinase assays with anti-Xatr and control immunoprecipitates that were prepared exactly as described in Figure 4. (B) Phosphorylation of Xchk1 by recombinant human ATR. Wild-type (Wt) or kinase-inactive (Mut) Flag-tagged ATR was isolated from 293T cells as described in Materials and Methods. Each ATR preparation (Wt or Mut) was aliquoted into nine samples, eight of which were used in kinase assays with GST-Xchk1(306–352), GST-Xchk1(347–374), and the indicated SQ/TQ mutant fusion proteins as substrates. Immunoblotting with anti-Flag antibodies indicated that the amount of Flag-tagged ATR protein (Wt or Mut) in each preparation was comparable.
Figure 6
Phosphorylation of the SQ/TQ domain of Xchk1 in Xenopus egg extracts. (A) Modification of wild-type (WT) and various mutant 35S-labeled Xchk1 proteins in egg extracts. The indicated 35S-labeled Xchk1 proteins (WT, T314A, S344A, T314A/S344A, S356A, S365A, S356A/S365A, and 4AQ) were prepared in reticulocyte lysates as described (Kumagai et al. 1998) and incubated in interphase extracts containing sperm nuclei (1000/μL) in the absence (−) or presence (+) of 100 μg/mL aphidicolin. (B) Characterization of anti-S344-p antibodies. Antibodies that recognize phosphorylated Ser 344 were produced as described in Materials and Methods. The antibodies were tested by immunoblotting a peptide containing phosphorylated Ser 344 (S344-p) and the corresponding nonphosphorylated version of the same peptide (S344) that were spotted onto nitrocellulose (100 and 200 ng of each peptide were used). (C) Detection of phosphorylated Xchk1 with anti-S344-p antibodies. Xchk1-WT-GST-His6 (lanes 1,2) and Xchk1-S344A-GST-His6 (lanes 3,4) were incubated in egg extracts in the absence (lanes 1,3) or presence of aphidicolin (lanes 2,4). After 90 min, the recombinant Xchk1 proteins were reisolated with glutathione agarose and immunoblotted with either anti-GST antibodies (top panel) or anti-S344-p antibodies (bottom panel).
Figure 7
The Xchk1-4AQ mutant is defective in mediating the DNA replication checkpoint. (A) Xenopus egg extracts (lane 1) were immunodepleted with control antibodies (lane 2) or anti-Xchk1 antibodies (lanes 3–5). Next, no recombinant protein (lane 3), Xchk1-WT-GST-His6 (lane 4), or Xchk1-4AQ-GST-His6 (lane 5) was added back to the Xchk1-depleted extracts. The extracts were analyzed by immunoblotting with anti-Xchk1 antibodies. (B) Egg extracts lacking endogenous Xchk1 but containing Xchk1-WT-GST-His6 (filled circles), Xchk1–4AQ-GST-His6 (filled triangles), or no additional recombinant protein (filled squares) were activated with CaCl2 before the addition of sperm nuclei (1000 nuclei/μL) and aphidicolin (100 μg/mL). The timing of nuclear envelope breakdown (NEB) was monitored by microscopy. Half-maximal NEB occurred at 90 min in control extracts lacking aphidicolin.
Figure 8
Model for checkpoint signaling pathways in the Xenopus egg extract system.
Similar articles
- Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts.
Kumagai A, Dunphy WG. Kumagai A, et al. Mol Cell. 2000 Oct;6(4):839-49. doi: 10.1016/s1097-2765(05)00092-4. Mol Cell. 2000. PMID: 11090622 - Activation of Xenopus Chk1 by mutagenesis of threonine-377.
Wang SX, Dunphy WG. Wang SX, et al. FEBS Lett. 2000 Dec 29;487(2):277-81. doi: 10.1016/s0014-5793(00)02370-x. FEBS Lett. 2000. PMID: 11150524 - The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts.
Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. Kumagai A, et al. J Cell Biol. 1998 Sep 21;142(6):1559-69. doi: 10.1083/jcb.142.6.1559. J Cell Biol. 1998. PMID: 9744884 Free PMC article. - Chk1 in the DNA damage response: conserved roles from yeasts to mammals.
Chen Y, Sanchez Y. Chen Y, et al. DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1025-32. doi: 10.1016/j.dnarep.2004.03.003. DNA Repair (Amst). 2004. PMID: 15279789 Review. - The Xenopus cell cycle: an overview.
Philpott A, Yew PR. Philpott A, et al. Methods Mol Biol. 2005;296:95-112. doi: 10.1385/1-59259-857-9:095. Methods Mol Biol. 2005. PMID: 15576928 Review.
Cited by
- Study of the DNA damage checkpoint using Xenopus egg extracts.
Willis J, DeStephanis D, Patel Y, Gowda V, Yan S. Willis J, et al. J Vis Exp. 2012 Nov 5;(69):e4449. doi: 10.3791/4449. J Vis Exp. 2012. PMID: 23149695 Free PMC article. - Preferential binding of ATR protein to UV-damaged DNA.
Unsal-Kaçmaz K, Makhov AM, Griffith JD, Sancar A. Unsal-Kaçmaz K, et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):6673-8. doi: 10.1073/pnas.102167799. Proc Natl Acad Sci U S A. 2002. PMID: 12011431 Free PMC article. - Regulation of Chk1 kinase by autoinhibition and ATR-mediated phosphorylation.
Katsuragi Y, Sagata N. Katsuragi Y, et al. Mol Biol Cell. 2004 Apr;15(4):1680-9. doi: 10.1091/mbc.e03-12-0874. Epub 2004 Feb 6. Mol Biol Cell. 2004. PMID: 14767054 Free PMC article. - Human RPA phosphorylation by ATR stimulates DNA synthesis and prevents ssDNA accumulation during DNA-replication stress.
Vassin VM, Anantha RW, Sokolova E, Kanner S, Borowiec JA. Vassin VM, et al. J Cell Sci. 2009 Nov 15;122(Pt 22):4070-80. doi: 10.1242/jcs.053702. Epub 2009 Oct 20. J Cell Sci. 2009. PMID: 19843584 Free PMC article. - DNA damage tolerance: a double-edged sword guarding the genome.
Ghosal G, Chen J. Ghosal G, et al. Transl Cancer Res. 2013;2(3):107-129. doi: 10.3978/j.issn.2218-676X.2013.04.01. Transl Cancer Res. 2013. PMID: 24058901 Free PMC article.
References
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
- Blasina A, de Weyer IV, Laus MC, Luyten WH, Parker AE, McGowan CH. A human homolog of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999a;9:1–10. - PubMed
- Blasina A, Price BD, Turenne GA, McGowan CH. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 1999b;9:1135–1138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous