Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons - PubMed (original) (raw)
Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons
A Rao et al. J Neurosci. 2000.
Abstract
To determine whether presynaptic input is necessary for postsynaptic differentiation, we isolated hippocampal neurons in microisland culture and thus deprived pyramidal cells of GABA input and GABAergic neurons of glutamate input. We find that glutamate input is necessary for clustering the AMPA-type glutamate receptor but not for clustering the NMDA receptor or the associated PSD-95 family scaffold in GABAergic cells; GABA input is not necessary for clustering the GABA(A) receptor or gephyrin in pyramidal cells. Isolated neurons showed a surprising mismatch of presynaptic and postsynaptic components. For example, in isolated pyramidal neurons, although GABA(A) receptor clusters covered <4% of the dendritic surface and presynaptic boutons covered <12%, a full two-thirds of the GABA(A) receptor clusters were localized inappropriately opposite the non-GABAergic, presumed glutamatergic, terminals. Furthermore, inhibitory and excitatory postsynaptic components were segregated into separate clusters in isolated cells and apposed to separate boutons of a single axon. Thus, GABA(A) receptors were clustered opposite some terminals, whereas NMDA receptors were clustered opposite other terminals of a single axon. These results suggest the involvement of a synaptogenic signal common to glutamate and GABA synapses that permits experimentally induced mismatching of presynaptic and postsynaptic components in isolated neurons, as well as a second specificity-conferring signal that mediates appropriate matching in mixed cultures.
Figures
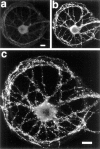
Fig. 1.
Effects of isolation on neuronal development.a, b, A pyramidal cell on a microisland stained with antibodies to MAP2 (a) and dephospho-tau (b) to label dendrites and axons, respectively. The axons grow profusely over the soma and dendrites, resulting in many contact sites where synapses could form. c, Pyramidal cell in contiguous culture labeled with an antibody to synaptophysin to show the density of synaptic vesicle clusters. d, Pyramidal island labeled with an antibody to synaptophysin showing a large number of puncta that represent presumptive autaptic sites. Scale bar, 10 μm.
Fig. 2.
GABAA receptors can form clusters on pyramidal cells in the absence of GABA input. An isolated pyramidal cell identified by the absence of GABA staining (a) and the presence of spiny clusters of the AMPA receptor (b) shows distinct clusters when labeled with an antibody to the β2,3 subunit of the GABAAreceptor (c). Scale bars: a, b, 10 μm; c, 10 μm.
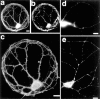
Fig. 3.
AMPA receptors are not clustered on GABAergic cells in the absence of glutamate input, but other components of excitatory postsynaptic sites are clustered. a–c, An isolated GABAergic cell identified by positive labeling for GABA (a) and GAD (b) shows no clusters when labeled with an antibody to the GluR1 subunit of the AMPA receptor (c). GluR1 immunoreactivity is present in the cell body and dendrites but excluded from axons (note the thin GABA-positive processes that do not label for GluR1). d, An isolated GABAergic cell identified by positive labeling for GAD (data not shown) bears distinct clusters of PSD-95. e, An isolated GABAergic cell identified by positive labeling for GAD (data not shown) shows punctate labeling for the NR1 subunit of the NMDA receptor. Scale bars: a, b, 10 μm;c–e, 10 μm.
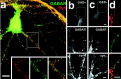
Fig. 4.
Mismatch of presynaptic and postsynaptic elements in pyramidal microislands. a, An isolated pyramidal cell immunolabeled with antibodies to the β2,3 subunit of the GABAA receptor (green) and synaptophysin (red) showing many appositions of the two (yellow). Inset, The_boxed region_ in a shown at double magnification in separate red and green panels as well as the red/green overlay.b, c, Comparison of triple-label immunolabeling for GAD (GAD), γ2 subunit of the GABAA receptor (GABAR), and synaptophysin (syn) in an innervated cell (b) and an isolated one (c). Immunolabeling for GAD (top panels) shows GABAergic presynaptic specializations in the innervated cell in b, whereas the isolated pyramidal cell in c has none. The boxed regions are shown enlarged below in middle panels (GABAR) and in bottom panels(syn). Both innervated and isolated cells have clusters of the γ2 subunit of the GABAA receptor apposed to clusters of synaptic vesicles defined by puncta of synaptophysin immunolabel (arrows). d, Region of an isolated pyramidal cell immunolabeled with antibodies to gephyrin (green, top panel) and synaptophysin (red, middle panel). Many of the larger gephyrin clusters are apposed to synaptophysin (yellow, in the bottom overlay panel). Scale bars: a, 10 μm;inset (shown in a), 20 μm; b, c, GAD panels, 10 μm; b, c, GABAR and synaptophysin panels, 5 μm; d, 5 μm.
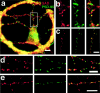
Fig. 5.
Mismatch of presynaptic and postsynaptic elements in GABAergic microislands. a, An isolated GABAergic cell immunolabeled with antibodies to PSD-95 (green) and GAD (red) shows a large degree of apposition of the two (yellow). b, c, A twofold magnification of the boxed region in a(b) and of a similar region from another isolated cell (c) is shown with PSD-95 in_green_ (left panel), GAD in red(middle panel), and in the red/green overlay(right panel). d, Similar appositions are seen in an isolated GABAergic cell double-labeled for GAD (red) and the NR2A subunit of the NMDA receptor (green). e, Extensive apposition in an isolated GABAergic cell double-labeled for GAD (red) and the β2,3 subunit of the GABAA receptor (green) is shown. Scale bars: a, 10 μm;d, e, 5 μm.
Fig. 6.
Segregation of inhibitory and excitatory postsynaptic specializations in isolated pyramidal cells.a, Phase image of an isolated pyramidal cell. Immunolabeling in the boxed region is shown at higher magnification in b. b, An isolated pyramidal cell immunolabeled with antibodies to the γ2 subunit of the GABAA receptor (GABAR), the NR1 subunit of the NMDA receptor (NR1), and synaptophysin (syn). An arrow indicates GABAA receptor clusters at synaptic sites without NR1. An_arrowhead_ indicates NR1 clusters at synaptic sites without GABAA receptor. c, An isolated pyramidal cell immunolabeled with antibodies to the β2,3 subunit of the GABAA receptor (GABAR), PSD-95 (PSD-95), and synaptophysin (syn). An_arrow_ indicates GABAA receptor clusters at synaptic sites without PSD-95. An arrowhead indicates PSD-95 clusters at synaptic sites without GABAA receptor.d, An isolated pyramidal cell immunolabeled with antibodies to the β2,3 subunit of the GABAA receptor (GABAR) and the GluR1 subunit of the AMPA receptor (GluR1). An arrow indicates GABAA receptor clusters at synaptic sites without GluR1. An_arrowhead_ indicates GluR1 clusters at synaptic sites without GABAA receptor. Scale bars: a, 20 μm; b–d, 5 μm.
Fig. 7.
Blockade of postsynaptic receptors does not affect the degree of mismatch. Contiguous cultures grown under conditions of spontaneous activity (a) or with chronic blockade of AMPA, NMDA, and GABAA receptors as well as of voltage-gated sodium channels (b) were immunostained for GAD as a presynaptic marker of GABAergic synapses (red) and PSD-95 as a postsynaptic marker of glutamatergic synapses (green). Mismatched presynaptic and postsynaptic appositions (arrows) are rare under both conditions. Scale bar, 10 μm.
Fig. 8.
Summary of results leading to the hypothesis that a common signal must exist at excitatory and inhibitory synaptic sites that permits the observed level of synaptic mismatch. In multi-innervated cells, GABA-containing presynaptic specializations are specifically apposed to postsynaptic specializations containing clusters of GABA receptor as well as of gephyrin and other scaffolding molecules for inhibitory synapses, and glutamate-containing presynaptic specializations are apposed to postsynaptic clusters of AMPA- and NMDA-type glutamate receptors as well as scaffolding proteins such as PSD-95 family members. In contrast, in isolated GABAergic cells GABA-containing presynaptic specializations also become apposed to clusters of the NMDA receptor and PSD-95, and in isolated pyramidal neurons glutamatergic presynaptic specializations also become apposed to clusters of the GABA receptor and gephyrin scaffolds. The existence of such mismatched appositions suggests the hypothesis that there is an element common to GABA and glutamate synapses that permits mismatch.
Similar articles
- GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons.
Christie SB, Miralles CP, De Blas AL. Christie SB, et al. J Neurosci. 2002 Feb 1;22(3):684-97. doi: 10.1523/JNEUROSCI.22-03-00684.2002. J Neurosci. 2002. PMID: 11826098 Free PMC article. - Differential regulation of GABA(A) receptor and gephyrin postsynaptic clustering in immature hippocampal neuronal cultures.
Studler B, Sidler C, Fritschy JM. Studler B, et al. J Comp Neurol. 2005 Apr 11;484(3):344-55. doi: 10.1002/cne.20472. J Comp Neurol. 2005. PMID: 15739236 - GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development.
Huang ZJ, Scheiffele P. Huang ZJ, et al. Curr Opin Neurobiol. 2008 Feb;18(1):77-83. doi: 10.1016/j.conb.2008.05.008. Epub 2008 May 29. Curr Opin Neurobiol. 2008. PMID: 18513949 Free PMC article. Review. - Cell type specificity of GABA(A) receptor mediated signaling in the hippocampus.
Semyanov A. Semyanov A. Curr Drug Targets CNS Neurol Disord. 2003 Aug;2(4):240-7. doi: 10.2174/1568007033482832. Curr Drug Targets CNS Neurol Disord. 2003. PMID: 12871034 Review.
Cited by
- Nicotinic alpha 7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins.
Kawai H, Zago W, Berg DK. Kawai H, et al. J Neurosci. 2002 Sep 15;22(18):7903-12. doi: 10.1523/JNEUROSCI.22-18-07903.2002. J Neurosci. 2002. PMID: 12223543 Free PMC article. - Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses.
Huh KH, Fuhrer C. Huh KH, et al. Mol Neurobiol. 2002 Feb;25(1):79-112. doi: 10.1385/MN:25:1:079. Mol Neurobiol. 2002. PMID: 11890459 Review. - Slow actions of neuroactive steroids at GABAA receptors.
Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Shu HJ, et al. J Neurosci. 2004 Jul 28;24(30):6667-75. doi: 10.1523/JNEUROSCI.1399-04.2004. J Neurosci. 2004. PMID: 15282269 Free PMC article. - New ways to print living cells promise breakthroughs for engineering complex tissues in vitro.
Withers GS. Withers GS. Biochem J. 2006 Mar 1;394(Pt 2):e1-2. doi: 10.1042/bj20060137. Biochem J. 2006. PMID: 16479619 Free PMC article. - Control of GABA Release at Mossy Fiber-CA3 Connections in the Developing Hippocampus.
Safiulina VF, Caiati MD, Sivakumaran S, Bisson G, Migliore M, Cherubini E. Safiulina VF, et al. Front Synaptic Neurosci. 2010 Feb 22;2:1. doi: 10.3389/neuro.19.001.2010. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423487 Free PMC article.
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
- Antonov I, Chang S, Zakharenko S, Popov SV. Distribution of neurotransmitter secretion in growing axons. Neuroscience. 1999;90:975–984. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous