Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development - PubMed (original) (raw)
Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development
J R O'Kusky et al. J Neurosci. 2000.
Abstract
The in vivo actions of insulin-like growth factor-I (IGF-I) on the growth and development of the hippocampal dentate gyrus were investigated in transgenic mice that overexpress IGF-I postnatally in the brain and in normal nontransgenic littermate controls. Stereological analyses of the dentate gyrus were performed by light and electron microscopy on days 7, 14, 21, 28, 35, and 130 to determine postnatal changes in the numerical density and total number of neurons and synapses. The volumes of both the granule cell layer and the molecular layer of the dentate gyrus were significantly increased by 27-69% in transgenic mice after day 7, with the greatest relative increases occurring by day 35. Although the numerical density of neurons in the granule cell layer did not differ significantly between transgenic and control mice at any age studied, the total number of neurons was significantly greater in transgenic mice by 29-61% beginning on day 14. The total number of synapses in the molecular layer was significantly increased by 42-105% in transgenic mice from day 14 to day 130. A transient increase in the synapse-to-neuron ratio was found in transgenic mice at postnatal days 28 and 35 but not at day 130. This finding indicates a disproportionate increase in synaptogenesis, exceeding that expected for the observed increase in neuron number. Our results demonstrate that IGF-I overexpression produces persistent increases in the total number of neurons and synapses in the dentate gyrus, indicating that IGF-I promotes both neurogenesis and synaptogenesis in the developing hippocampus in vivo.
Figures
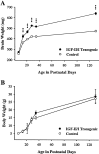
Fig. 1.
Postnatal changes in brain weight (A) and body weight (B) for IGF-II/IGF-I transgenic mice and normal littermate controls. Values are presented as the mean ± SEM for three to four mice in each group. ANOVA for brain weight revealed significant main effects for groups (F = 277; p < 0.001), ages (F = 241; p < 0.001), and group × age interactions (F = 17.19;p < 0.001). ***p < 0.001 for individual paired comparisons between transgenic and control mice. ANOVA for body weight revealed a significant main effect for age (F = 120; p < 0.001) but no significant main effects for groups (F = 0.92;p = 0.35) or group × age interactions (F = 0.78; p = 0.57).
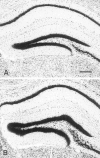
Fig. 2.
Representative sections of the hippocampal dentate gyrus in (A) normal control and (B) IGF-II/IGF-I transgenic mice at postnatal day 35. Serial frozen sections (30 μm) through the hippocampus were stained for Nissl substance with aqueous thionine. Scale bar, 250 μm.
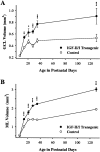
Fig. 3.
Postnatal changes in the total volumes of the GCL (A) and the ML (B) for IGF-II/IGF-I transgenic mice and normal littermate controls. Values are presented as the mean ± SEM for three to four mice in each group. ANOVA for volume of the GCL revealed significant main effects for groups (F = 138; p < 0.001), ages (F = 60.86; p < 0.001), and group × age interactions (F = 8.59;p < 0.001). ANOVA for volume of the ML revealed significant main effects for groups (F = 275;p < 0.001), ages (F = 242; p < 0.001), and group × age interactions (F = 22.61; p < 0.001). *p < 0.05; **p < 0.01; ***p < 0.001 for individual paired comparisons between transgenic and control mice.
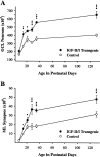
Fig. 4.
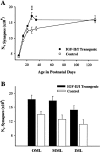
Postnatal changes in the total numbers of neurons in the GCL (A) and the total of synapses in the ML (B) for IGF-II/IGF-I transgenic mice and normal littermate controls. Values are presented as the mean ± SEM for three to four mice in each group. ANOVA for total neurons in the GCL revealed significant main effects for groups (F = 118; p < 0.001), ages (F = 50.44; p < 0.001), and group × age interactions (F = 5.88;p < 0.001). ANOVA for total synapses in the ML revealed significant main effects for groups (F = 140; p < 0.001), ages (F = 131; p < 0.001), and group × age interactions (F = 11.25; p < 0.001). *p < 0.05; **p < 0.01; ***p < 0.001 for individual paired comparisons between transgenic and control mice.
Fig. 5.
Epon-embedded semithin sections (0.7 μm) stained with toluidine blue through the GCL of the dentate gyrus in normal control (A) and IGF-II/IGF-I transgenic mice (B) at postnatal day 130. Scale bar, 20 μm.
Fig. 6.
Postnatal changes in the_N_V of synapses in the ML (A) for IGF-II/IGF-I transgenic mice and normal littermate controls. Values are presented as the mean ± SEM for three to four mice in each group. ANOVA for the_N_V of synapses revealed significant main effects for groups (F = 15.43;p < 0.001), ages (F = 62.55;p < 0.001), and group × age interactions (F = 2.82; p < 0.05). *p < 0.05; ***p < 0.001 for individual paired comparisons between transgenic and control mice. The_N_V of synapses in the OML, MML, and IML (B) in transgenic and control mice on postnatal day 28. Values are given as the mean ± SEM for four mice in each group. ANOVA for the _N_V of synapses in individual laminae revealed a significant main effect for group (F = 21.58; p < 0.001) but not for laminae (F = 3.04; p = 0.07) or group × laminae interactions (F = 0.15; p = 0.86).
Fig. 7.
Electron micrographs from the ML of the dentate gyrus in IGF-II/IGF-I transgenic (A–C,E) and control (D) mice. The vast majority of synapses formed asymmetric axospinous contacts (A) in both transgenic and control mice, identified by the presence of a prominent postsynaptic density and predominantly spherical vesicles in the presynaptic axon terminal (B). Symmetric axodendritic contacts (C) were observed less frequently, identified by the presence of differentiated presynaptic and postsynaptic membranes without a prominent postsynaptic density and pleomorphic vesicles in the presynaptic axon terminal. Electron micrographs of the neuropil in the OML of normal control (D) and IGF-II/IGF-I transgenic (E) mice at postnatal day 28. Scale bars: A, 0.5 μm; B, C, 0.2 μm; D, E, 3 μm.
Fig. 8.
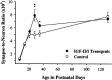
Postnatal changes in the synapse-to-neuron ratio in IGF-II/IGF-I transgenic mice and normal controls. Values are given as the mean ± SEM for three to four mice in each group. ANOVA revealed significant main effects for groups (F = 4.88; p < 0.05), ages (F = 38.31; p < 0.001), and group × age interactions (F = 3.47; p < 0.05). *p < 0.05; **p < 0.01 for individual paired comparisons between transgenic and control mice.
Similar articles
- Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus.
Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Poe BH, et al. Neuroscience. 2001;107(2):231-8. doi: 10.1016/s0306-4522(01)00341-4. Neuroscience. 2001. PMID: 11731097 - Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus.
Rahimi O, Claiborne BJ. Rahimi O, et al. Prog Brain Res. 2007;163:167-81. doi: 10.1016/S0079-6123(07)63010-6. Prog Brain Res. 2007. PMID: 17765718 Review. - New interneurons in the adult neocortex: small, sparse, but significant?
Cameron HA, Dayer AG. Cameron HA, et al. Biol Psychiatry. 2008 Apr 1;63(7):650-5. doi: 10.1016/j.biopsych.2007.09.023. Epub 2007 Dec 11. Biol Psychiatry. 2008. PMID: 18067877 Free PMC article. Review.
Cited by
- Reversal of opiate-induced apoptosis by human recombinant growth hormone in murine foetus primary hippocampal neuronal cell cultures.
Svensson AL, Bucht N, Hallberg M, Nyberg F. Svensson AL, et al. Proc Natl Acad Sci U S A. 2008 May 20;105(20):7304-8. doi: 10.1073/pnas.0802531105. Epub 2008 May 12. Proc Natl Acad Sci U S A. 2008. PMID: 18474854 Free PMC article. - Early impoverished environment delays the maturation of cerebral cortex.
Narducci R, Baroncelli L, Sansevero G, Begenisic T, Prontera C, Sale A, Cenni MC, Berardi N, Maffei L. Narducci R, et al. Sci Rep. 2018 Jan 19;8(1):1187. doi: 10.1038/s41598-018-19459-y. Sci Rep. 2018. PMID: 29352131 Free PMC article. - Dendritic spine dysgenesis in Rett syndrome.
Xu X, Miller EC, Pozzo-Miller L. Xu X, et al. Front Neuroanat. 2014 Sep 10;8:97. doi: 10.3389/fnana.2014.00097. eCollection 2014. Front Neuroanat. 2014. PMID: 25309341 Free PMC article. Review. - The Effect of Plasma Rich in Growth Factors on Microglial Migration, Macroglial Gliosis and Proliferation, and Neuronal Survival.
Ruzafa N, Pereiro X, Fonollosa A, Araiz J, Acera A, Vecino E. Ruzafa N, et al. Front Pharmacol. 2021 Feb 26;12:606232. doi: 10.3389/fphar.2021.606232. eCollection 2021. Front Pharmacol. 2021. PMID: 33716738 Free PMC article. - The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease.
Araki T, Ikegaya Y, Koyama R. Araki T, et al. Eur J Neurosci. 2021 Sep;54(5):5880-5901. doi: 10.1111/ejn.14969. Epub 2020 Sep 21. Eur J Neurosci. 2021. PMID: 32920880 Free PMC article. Review.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–336. - PubMed
- Angevine JB. Time of neuron origin in the hippocampal region: an autoradiographic study in the mouse. Exp Neurol [Suppl] 1965;2:1–70. - PubMed
- Bach MA, Shen-Orr Z, Lower WL, Roberts CT, LeRoith D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Mol Brain Res. 1991;10:43–48. - PubMed
- Baker J, Liu J, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases