Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons - PubMed (original) (raw)
Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons
B D Bennett et al. J Neurosci. 2000.
Abstract
Neostriatal cholinergic interneurons produce spontaneous tonic firing in the absence of synaptic input. Perforated patch recording and whole-cell recording combined with calcium imaging were used in vitro to identify the intrinsic membrane properties underlying endogenous excitability. Spontaneous firing was driven by the combined action of a sodium current and the hyperpolarization-activated cation current (I(h)), which together ensured that there was no zero current point in the subthreshold voltage range. Blockade of sodium channels or I(h) established a stable subthreshold resting membrane potential. A tetrodotoxin-sensitive region of negative slope conductance was observed between approximately -60 mV and threshold (approximately -50 mV) and the h-current was activated at all subthreshold voltages. Calcium imaging experiments revealed that there was minimal calcium influx at subthreshold membrane potentials but that action potentials produced elevations of calcium in both the soma and dendrites. Spike-triggered calcium entry shaped the falling phase of the action potential waveform and activated calcium-dependent potassium channels. Blockade of big-conductance channels caused spike broadening. Application of apamin, which blocks small-conductance channels, abolished the slow spike afterhyperpolarization (AHP) and caused a transition to burst firing. In the absence of synaptic input, a range of tonic firing patterns are observed, suggesting that the characteristic spike sequences described for tonically active cholinergic neurons (TANs) recorded in vivo are intrinsic in origin. The pivotal role of the AHP in regulating spike patterning indicates that burst firing of TANs in vivo could arise from direct or indirect modulation of the AHP without requiring phasic synaptic input.
Figures
Fig. 1.
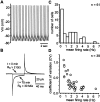
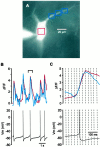
Firing rates and patterns of cholinergic cells.A, Example of a spike train recorded from a tonically active cholinergic neuron using the perforated patch configuration 45 min after seal formation. B, Averaged responses (50–100 trials) to a 2 mV voltage step recorded immediately after seal formation and 75 min later. C, The firing rates of cholinergic cells were skewed toward lower values with the mean and median falling between 1 and 2 Hz. D, A plot of the CV versus the firing rate indicates that cholinergic cells that fire more rapidly exhibit more regular spike trains.
Fig. 2.
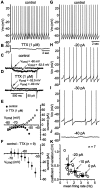
The role of sodium in spontaneous tonic firing.A, Spontaneous firing was observed in the majority of cholinergic interneurons. B, Application of TTX (1 μ
m
) prevented action potential generation and established a stable subthreshold membrane potential (approximately −60 mV). C, Stepping the membrane from a holding potential of −60 to −52.5 mV elicited a slowly developing inward current that persisted throughout the pulse. The dashed line indicates the zero current point. D, After TTX (1 μ
m
) treatment, the inward current produced by the depolarizing voltage step was absent, and a small outward current was observed. Additionally, the zero current point was shifted to −60 mV. E, The I–V plot for a range of voltage steps (_V_cmd) shows that, under control conditions, there is a region of negative slope conductance at potentials positive to −60 mV and no zero current point in the subthreshold voltage range. The inward current generated by depolarizing steps was blocked by TTX. F, Examination of the TTX-sensitive current that was obtained by subtraction for nine neurons and pooled (mean ± SD). G–J, Spiking rate was reduced by injection of constant negative current and failed to reveal any subthreshold oscillation in the absence of action potential generation. The firing pattern was related to firing rate, and spike trains became increasingly irregular at lower rates. K, For seven neurons exhibiting tonic, regular spiking (2.45–4.03 Hz; CV, 0.10–0.19), the firing rate and pattern was measured for control (0 pA) and during steady injection of −10 and −20 pA (open circles). Pooled data (filled circles; mean ± SD) confirmed that firing pattern was a function of spiking rate.
Fig. 3.
_I_h is required for spontaneous firing. A, Tonic firing observed under control conditions. B, Application of cesium (3 m
m
) produced a profound decrease in the firing rate and an accompanying increase in the irregularity of the spike train.C, After washout of cesium, the firing rate and pattern were restored to control values. D, Injection of negative current produced an initial hyperpolarization followed by a sag in the membrane potential caused by activation of_I_h. Cesium abolished the sag, confirming that I_h was blocked. E, Stepping from a holding potential of −60 to −80 mV evoked a slowly developing inward current. F, Application of cesium reduced the amplitude of the inward current measured at the end of the pulse and shifted the zero current point. G, The_I–V plot for a range of voltage steps (_V_cmd) from a holding potential of −60 mV reveals that cesium blocks an inward current that is activated throughout the subthreshold voltage range. Cesium also established a zero current point at approximately −65 mV in this neuron.H, The cesium-sensitive current was obtained by subtraction (n = 5) and exhibited the voltage dependence expected for _I_h.
Fig. 4.
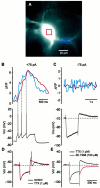
Voltage dependence of calcium influx.A, Fura-2-loaded cholinergic neuron at an excitation wavelength of 380 nm. The red box over the soma and the_blue boxes_ over the dendrites are the areas from which the fluorescence signals were measured. B, Examination of the response to current injection (+75 pA) revealed that each spike was associated with a calcium elevation in both the soma and dendrites.C, Injection of negative current (−75 pA) produced a hyperpolarization and a subsequent sag in the membrane potential attributable to activation of _I_h. After termination of the current pulse, a rebound in the membrane potential was observed. There was no detectable alteration in the fluorescence signal either in response to the hyperpolarization or the rebound. Calcium signals illustrated B and C are individual sweeps. D, Under control conditions, injection of negative current (−200 pA) produced a hyperpolarization and a subsequent sag in the membrane potential, which was followed by a rebound in the membrane potential after termination of the pulse. Application of TTX (1 μ
m
) reduced the amplitude the rebound. E, Blockade of _I_heliminated the sag during membrane hyperpolarization and abolished the rebound.
Fig. 5.
Calcium dynamics during spontaneous firing.A, Fluorescence image (380 nm excitation) of a cholinergic neuron loaded with fura-2 during conventional whole-cell recording. The colored boxes are the locations from which the fluorescence measurements were obtained. B, Simultaneous fluorescence and electrical measurements during spontaneous firing revealed concurrent spike-triggered elevations in calcium concentration in the soma and primary dendrite (bracket indicates data shown in C).C, Examination of a single spike and the associated calcium transient during spontaneous firing confirms that elevations in intracellular calcium are primarily spike-triggered with minimal calcium influx occurring during the depolarizing ramp to action potential threshold. The dashed lines demarcate the individual frames (30 msec) for the fluorescence data. Calcium signals illustrated B and C are individual sweeps.
Fig. 6.
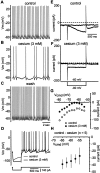
Calcium is critical for tonic firing.A, Spontaneous tonic firing. B, Application of cadmium (400 μ
m
) converted tonic spiking to rhythmic bursting, during which clusters of spikes were generated in rapid succession and separated by slow subthreshold oscillations in the membrane potential. C, Stepping from a holding potential of −60 to −80 mV activated the slowly developing h-current.D, After calcium channel blockade, the same voltage step produced a smaller current and was accompanied by a shift in the holding current at −60 mV. E, The _I–V_plot for this neuron generated using a range of voltage steps (_V_cmd) indicates that there has been a reduction in a subthreshold inward current. F, The current that was reduced by calcium channel blockade was obtained by subtraction and pooled (n = 5;symbols indicate mean ± SD) G, Under control conditions, cholinergic neurons generate relatively broad spikes that exhibit a shoulder on the falling phase. Calcium channel blockade produced a pronounced narrowing of the spike and abolished the shoulder. H, Application of cadmium abolished the slow AHP that normally follows individual spikes.
Fig. 7.
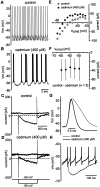
Calcium regulates the firing properties of cholinergic neurons. A, In response to depolarizing current injection (+40 pA; left), cholinergic cells generated spikes that were separated by a slow AHP. Injection of larger amplitude currents (+140 pA; right) evoked repetitive spiking with minimal spike frequency adaptation. B, After application of cadmium (400 μ
m
), the same cell generates many more spikes in response to a given current injection and spike-frequency adaptation. C, Calcium channel blockade produces a profound increase in the slope of the f–I_relationship (left). The instantaneous_f–I plot (+140 pA; right) shows a pronounced increase in the initial firing rate and substantial spike frequency adaptation after blockade of calcium channels.D, Pooled data (n = 13, 9 cadmium, 4 cobalt; symbols indicate mean ± SD) confirm that calcium channel blockade profoundly alters both the _f–I_and instantaneous f–I relationships.
Fig. 8.
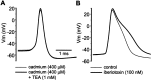
Spike-triggered calcium entry activates BK channels, which contribute to repolarization. A, Application of TEA (1 m
m
) after cadmium treatment had no discernable effect on action potential width. B, In the presence of calcium-containing ACSF, spike width was significantly broadened after treatment with TEA or iberiotoxin (100 n
m
).
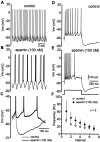
Fig. 9.
Calcium-activated SK channels underlie the AHP and regulate spike patterning. A, Under control conditions, spontaneous tonic firing was observed. B, Application of apamin (100 n
m
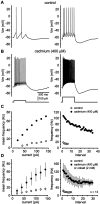
), which blocks calcium-dependent SK channels, converted tonic firing to rhythmic bursting.C, The AHP after individual spikes was completely blocked by apamin, but between bursts, there was a large slow hyperpolarization. D, Injection of depolarizing current elicited a train of spikes that displayed spike frequency adaptation.E, After application of apamin, the same current pulse evoked spiking with a higher initial firing rate but with much more pronounced adaptation and resulted in spike cessation before termination of the current pulse. F, Apamin produced an elevation in the instantaneous firing rate and more pronounced spike-frequency adaptation (n = 5;symbols indicate mean ± SD).
Similar articles
- Medium afterhyperpolarization and firing pattern modulation in interneurons of stratum radiatum in the CA3 hippocampal region.
Savić N, Pedarzani P, Sciancalepore M. Savić N, et al. J Neurophysiol. 2001 May;85(5):1986-97. doi: 10.1152/jn.2001.85.5.1986. J Neurophysiol. 2001. PMID: 11353015 - Iberiotoxin-sensitive large conductance Ca2+ -dependent K+ (BK) channels regulate the spike configuration in the burst firing of cerebellar Purkinje neurons.
Haghdoost-Yazdi H, Janahmadi M, Behzadi G. Haghdoost-Yazdi H, et al. Brain Res. 2008 May 30;1212:1-8. doi: 10.1016/j.brainres.2008.03.030. Epub 2008 Mar 27. Brain Res. 2008. PMID: 18439989 - Calcium-activated SK channels influence voltage-gated ion channels to determine the precision of firing in globus pallidus neurons.
Deister CA, Chan CS, Surmeier DJ, Wilson CJ. Deister CA, et al. J Neurosci. 2009 Jul 1;29(26):8452-61. doi: 10.1523/JNEUROSCI.0576-09.2009. J Neurosci. 2009. PMID: 19571136 Free PMC article. - Cav1.3 Channels as Key Regulators of Neuron-Like Firings and Catecholamine Release in Chromaffin Cells.
Vandael DH, Marcantoni A, Carbone E. Vandael DH, et al. Curr Mol Pharmacol. 2015;8(2):149-61. doi: 10.2174/1874467208666150507105443. Curr Mol Pharmacol. 2015. PMID: 25966692 Free PMC article. Review. - Signal processing by T-type calcium channel interactions in the cerebellum.
Engbers JD, Anderson D, Zamponi GW, Turner RW. Engbers JD, et al. Front Cell Neurosci. 2013 Nov 27;7:230. doi: 10.3389/fncel.2013.00230. Front Cell Neurosci. 2013. PMID: 24348329 Free PMC article. Review.
Cited by
- Ionic and morphological contributions to the variable gain of membrane responses in layer 2/3 pyramidal neurons of mouse primary visual cortex.
Routh BN, Brager DH, Johnston D. Routh BN, et al. J Neurophysiol. 2022 Oct 1;128(4):1040-1050. doi: 10.1152/jn.00181.2022. Epub 2022 Sep 21. J Neurophysiol. 2022. PMID: 36129187 Free PMC article. - A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem.
Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, Mena-Segovia J. Dautan D, et al. J Neurosci. 2014 Mar 26;34(13):4509-18. doi: 10.1523/JNEUROSCI.5071-13.2014. J Neurosci. 2014. PMID: 24671996 Free PMC article. - Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus.
Atherton JF, Kitano K, Baufreton J, Fan K, Wokosin D, Tkatch T, Shigemoto R, Surmeier DJ, Bevan MD. Atherton JF, et al. J Neurosci. 2010 Nov 24;30(47):16025-40. doi: 10.1523/JNEUROSCI.3898-10.2010. J Neurosci. 2010. PMID: 21106841 Free PMC article. - Chronic sleep fragmentation enhances habenula cholinergic neural activity.
Ge F, Mu P, Guo R, Cai L, Liu Z, Dong Y, Huang YH. Ge F, et al. Mol Psychiatry. 2021 Mar;26(3):941-954. doi: 10.1038/s41380-019-0419-z. Epub 2019 Apr 12. Mol Psychiatry. 2021. PMID: 30980042 Free PMC article. - Voltage-Gated Intrinsic Conductances Shape the Input-Output Relationship of Cortical Neurons in Behaving Primate V1.
Li B, Routh BN, Johnston D, Seidemann E, Priebe NJ. Li B, et al. Neuron. 2020 Jul 8;107(1):185-196.e4. doi: 10.1016/j.neuron.2020.04.001. Epub 2020 Apr 28. Neuron. 2020. PMID: 32348717 Free PMC article.
References
- Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982;296:746–749. - PubMed
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
- Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 1994;44:433–473. - PubMed
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994a;265:412–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources