The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN - PubMed (original) (raw)
The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN
A Alcamí et al. J Virol. 2000 Dec.
Abstract
Poxviruses encode a broad range of proteins that interfere with host immune functions, such as soluble versions of receptors for the cytokines tumor necrosis factor, interleukin-1 beta, gamma interferon (IFN-gamma), IFN-alpha/beta, and chemokines. These virus-encoded cytokine receptors have a profound effect on virus pathogenesis and enable the study of the role of cytokines in virus infections. The vaccinia virus (VV) Western Reserve gene B18R encodes a secreted protein with 3 immunoglobulin domains that functions as a soluble receptor for IFN-alpha/beta. We have found that after secretion B18R binds to both uninfected and infected cells. The B18R protein present at the cell surface maintains the properties of the soluble receptor, binding IFN-alpha/beta with high affinity and with broad species specificity, and protects cells from the antiviral state induced by IFN-alpha/beta. VV strain Wyeth expressed a truncated B18R protein lacking the C-terminal immunoglobulin domain. This protein binds IFN with lower affinity and retains its ability to bind to cells, indicating that the C-terminal region of B18R contributes to IFN binding. The replication of a VV B18R deletion mutant in tissue culture was restricted in the presence of IFN-alpha, whereas the wild-type virus replicated normally. Binding of soluble recombinant B18R to cells protected the cultures from IFN and allowed VV replication. This represents a novel strategy of virus immune evasion in which secreted IFN-alpha/beta receptors not only bind the soluble cytokine but also bind to uninfected cells and protect them from the antiviral effects of IFN-alpha/beta, maintaining the cells' susceptibility to virus infections. The adaptation of this soluble receptor to block IFN-alpha/beta activity locally will help VV to replicate in the host and spread in tissues. This emphasizes the importance of local effects of IFN-alpha/beta against virus infections.
Figures
FIG. 1
B18R binds specifically to cells. (A) B18R protein produced in the baculovirus system and radiolabeled with [35S]methionine (B18R) was incubated with medium (m) or with the indicated cell lines at 4°C for 2 to 3 h as described in Materials and Methods. Cell lines were preincubated (+) or not preincubated (−) with 10 ng of PMA/ml. The B18R protein bound to cells was analyzed by SDS-PAGE and fluorography. The arrowhead indicates the position of the B18R protein. The results shown are representative of three experiments. (B) B18R protein produced in the baculovirus system and radiolabeled with [35S]methionine was incubated with TK−143B cells for 3 h at 4°C in the presence or absence of increasing doses of medium (equivalent to 3 × 106 cells/ml) from insect cell cultures infected with recombinant baculovirus producing B18R or B15R. Cell monolayers were washed extensively, and the level of B18R protein bound to cells (duplicate samples; mean counts per minute ± standard deviation) was determined by TCA precipitation. The results shown are representative of three experiments.
FIG. 2
Complex formation of the B18R protein with IFN-α2. Supernatants from Sf cells infected with AcB18R and labeled metabolically with [35S]methionine and [35S]cysteine were incubated with or without IFN-α2 for 2 h at room temperature and then loaded onto sucrose density gradients (5 to 25% sucrose in PBS) and centrifuged as described in Materials and Methods. The original B18R sample (S) and fractions collected from the bottom of the gradient and precipitated with TCA were analyzed by SDS-PAGE. A fluorograph showing the distribution of radiolabeled B18R incubated or not with IFN-α2 is shown. The positions of the molecular size standards are indicated in kilodaltons. The open arrowhead indicates the position of B18R (48 kDa) in the polyacrylamide gel. The results shown are representative of two experiments.
FIG. 3
B18R attached to the cell surface binds IFN-α and prevents activation of IFN-mediated signal transduction. (A) Binding of IFN-α2 to cells coated with B18R. U937 or THP-1 cells (106) were preincubated for 2 h at 4°C with binding medium or supernatants from cultures of TK−143B cells that were mock infected or infected with VV WR or vΔB18R, equivalent to 105 cells. Subsequently, cells were washed twice by centrifugation with binding medium and incubated with 5 nM 125I-labeled IFN-α2 for 2 h at 4°C, and bound IFN levels were determined after centrifugation of cells through phthalate oil. The specificity of the 125I-labeled IFN-α2 binding to cellular receptors was determined in the presence of a 100-fold excess of IFN-α2. (B) Cell surface B18R inhibits signal transduction via the cellular IFN-α receptor. HeLa cells (5 × 107) were incubated with media or supernatants from baculovirus-infected cells, washed to remove soluble proteins, and then incubated with (+) or without (−) 500 U of human IFN-α/ml for 15 min at 37°C. Whole-cell extracts were prepared and immunoprecipitated with anti-JAK1 antiserum. Precipitated proteins were separated by SDS-PAGE (7.5% gel), transferred to nitrocellulose, and probed with the antiphosphotyrosine antibody 4G10 (anti-P-Y) (top panel). As a control for JAK1 expression, blots were stripped and reprobed with anti-JAK1 antibody (bottom panel). The positions of molecular size standards are indicated in kilodaltons.
FIG. 4
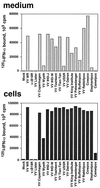
Expression of IFN binding activity at the surfaces of cells infected with orthopoxviruses. TK−143B cells were mock infected or infected at 1 PFU/cell with the indicated orthopoxviruses. At 12 h postinfection, cell monolayers were washed twice in binding medium and incubated for 2 h at 4°C with 1 nM 125I-labeled IFN-α2. Cells were washed twice with binding medium and lysed with 0.1% SDS, and the radioactivity bound to cells was determined (cells). The production of viral IFN-α/βRs secreted from the infected cultures was determined in a soluble binding assay with 125I-labeled IFN-α2 by precipitating ligand-receptor complexes with PEG and filtration through GF/C filters (medium).
FIG. 5
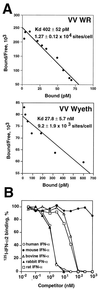
IFN binding properties of the B18R protein at the cell surface. (A) Scatchard analysis of binding of human 125I-labeled IFN-α2 to cell surface B18R. Mouse L929 cells (107) were infected with either VV strain WR or VV strain Wyeth at 10 PFU/cell for 12 h and then were incubated in 0.2 ml with different concentrations of 125I-labeled human IFN-α2. Specific binding was determined after centrifugation of cells through phthalate oil. Data were converted to the Scatchard coordinate system. (B) Species specificity of cell surface B18R binding; cross-competition of human 125I-labeled IFN-α2 binding to L929 cells infected with VV WR. 125I-labeled IFN-α2 (10 nM) was incubated with L929 cells (2 × 106) infected with VV strain WR at 10 PFU/cell for 12 h together with increasing concentrations of cold human recombinant IFN-α2 (open circles), mouse natural IFN-α (solid circles), bovine recombinant IFN-α1 (open triangles), rabbit natural type I IFN (solid triangles), and rat natural type I IFN (open squares) for 3 h at 4°C. 125I-labeled IFN-α2 that had bound to the cell surface was separated from free ligand by centrifugation through phthalate oil. The level of binding in the absence of competitor was 17,936 ± 186 cpm.
FIG. 6
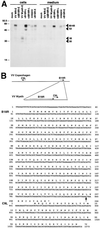
Characterization of B18R encoded by VV Wyeth. (A) Identification of the B18R protein by immunoprecipitation. TK−143B cells were mock infected or infected with the indicated viruses and pulse-labeled with 35S-labeled Promix and [35S]cysteine from 1 to 5 h postinfection (early [E]) or from 4 to 8 h postinfection (late [L]). Cells and media were dissociated with RIPA buffer and immunoprecipitated with anti-B18R Ig. Immunoprecipitates were analyzed by SDS-PAGE and fluorography. The position of B18R is indicated by arrowheads. Molecular sizes in kilodaltons are shown on the left. The results shown are representative of two experiments. (B) Nucleotide and amino acid sequences of the B18R ORF from VV Wyeth. The diagram shows the location of ORFs B19R (B18R in VV WR) and C9L in the genome of VV Copenhagen, and the location of these ORFs in VV Wyeth. The arrow after nucleotide 836 in the DNA sequence marks the site where the 3′ region of the C9L ORF was inserted into the B18R ORF in VV Wyeth.
FIG. 7
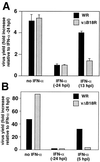
Replication of VV in tissue culture in the presence of IFN. Cultures of human FS2 cells (A) or rabbit RK13 cells (B) were infected with 200 PFU of VV WR or vΔB18R/well, and virus production was determined 48 h postinfection (hpi). Cultures were also treated with 500 U of human natural IFN-α (FS2 cells) or rabbit type I IFN (RK13 cells), added 24 h before infection or at the indicted time after infection. Virus yield is expressed as the fold increase relative to the virus production in cultures pretreated with IFN (duplicate samples, means ± standard deviations in the case of FS2 cells). The results shown are representative of two experiments.
FIG. 8
Treatment of cell monolayers with recombinant B18R protein blocks the antiviral effects mediated by IFN. (A) Confluent BSC-1 cells were infected with approximately 100 PFU of VV strain WR or vΔB18R for 2 h at 37°C. After removal of the virus inoculum, cells were overlaid with either medium or medium containing 106 cell equivalents of supernatant from AcB18R-infected cells and were incubated at 37°C for 4 h. Cell monolayers were then washed three times with medium to remove soluble proteins and were then overlaid with medium containing 1.5% (wt/vol carboxymethyl cellulose with (+IFN) or without (−IFN) 100 U of human IFN-α (Wellferon)/ml. After incubation for 48 h, cells were stained with 0.1% (wt/vol) crystal violet in 15% ethanol. (B) Virus production and plaque formation in FS2 cells. Human FS2 cells were left untreated or treated with 100 U of human natural IFN-α/ml 24 h before infection with vΔB18R. At 3 h postinfection, cultures were also incubated with medium from an equivalent number of insect cell cultures infected with AcB15R or AcB18R expressing high levels of recombinant B15R or B18R proteins, respectively. Cell monolayers were washed twice with medium, and the infection was continued in the presence of IFN-α (100 U/ml). At 48 h postinfection, virus levels in infected cells were determined by plaque assay, and the virus plaques were stained with 0.1% (wt/vol) crystal violet in 15% ethanol. Virus yield is expressed as the fold increase (triplicate samples, means ± standard deviations) relative to the virus production in cultures pretreated with IFN.
FIG. 8
Treatment of cell monolayers with recombinant B18R protein blocks the antiviral effects mediated by IFN. (A) Confluent BSC-1 cells were infected with approximately 100 PFU of VV strain WR or vΔB18R for 2 h at 37°C. After removal of the virus inoculum, cells were overlaid with either medium or medium containing 106 cell equivalents of supernatant from AcB18R-infected cells and were incubated at 37°C for 4 h. Cell monolayers were then washed three times with medium to remove soluble proteins and were then overlaid with medium containing 1.5% (wt/vol carboxymethyl cellulose with (+IFN) or without (−IFN) 100 U of human IFN-α (Wellferon)/ml. After incubation for 48 h, cells were stained with 0.1% (wt/vol) crystal violet in 15% ethanol. (B) Virus production and plaque formation in FS2 cells. Human FS2 cells were left untreated or treated with 100 U of human natural IFN-α/ml 24 h before infection with vΔB18R. At 3 h postinfection, cultures were also incubated with medium from an equivalent number of insect cell cultures infected with AcB15R or AcB18R expressing high levels of recombinant B15R or B18R proteins, respectively. Cell monolayers were washed twice with medium, and the infection was continued in the presence of IFN-α (100 U/ml). At 48 h postinfection, virus levels in infected cells were determined by plaque assay, and the virus plaques were stained with 0.1% (wt/vol) crystal violet in 15% ethanol. Virus yield is expressed as the fold increase (triplicate samples, means ± standard deviations) relative to the virus production in cultures pretreated with IFN.
Similar articles
- Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity.
Symons JA, Alcamí A, Smith GL. Symons JA, et al. Cell. 1995 May 19;81(4):551-60. doi: 10.1016/0092-8674(95)90076-4. Cell. 1995. PMID: 7758109 - Analysis of an interaction between the soluble vaccinia virus-coded type I interferon (IFN)-receptor and human IFN-alpha1 and IFN-alpha2.
Liptáková H, Kontseková E, Alcamí A, Smith GL, Kontsek P. Liptáková H, et al. Virology. 1997 May 26;232(1):86-90. doi: 10.1006/viro.1997.8527. Virology. 1997. PMID: 9185591 - Characterization of ATI, TK and IFN-alpha/betaR genes in the genome of the BeAn 58058 virus, a naturally attenuated wild Orthopoxvirus.
Marques JT, Trindade GD, Da Fonseca FG, Dos Santos JR, Bonjardim CA, Ferreira PC, Kroon EG. Marques JT, et al. Virus Genes. 2001 Dec;23(3):291-301. doi: 10.1023/a:1012521322845. Virus Genes. 2001. PMID: 11778697 - How Does Vaccinia Virus Interfere With Interferon?
Smith GL, Talbot-Cooper C, Lu Y. Smith GL, et al. Adv Virus Res. 2018;100:355-378. doi: 10.1016/bs.aivir.2018.01.003. Epub 2018 Feb 16. Adv Virus Res. 2018. PMID: 29551142 Review. - Receptors for gamma-interferon encoded by poxviruses: implications for the unknown origin of vaccinia virus.
Alcamí A, Smith GL. Alcamí A, et al. Trends Microbiol. 1996 Aug;4(8):321-6. doi: 10.1016/0966-842x(96)10051-2. Trends Microbiol. 1996. PMID: 8856871 Review.
Cited by
- The interferons and their receptors--distribution and regulation.
de Weerd NA, Nguyen T. de Weerd NA, et al. Immunol Cell Biol. 2012 May;90(5):483-91. doi: 10.1038/icb.2012.9. Epub 2012 Mar 13. Immunol Cell Biol. 2012. PMID: 22410872 Free PMC article. Review. - Removal of vaccinia virus genes that block interferon type I and II pathways improves adaptive and memory responses of the HIV/AIDS vaccine candidate NYVAC-C in mice.
Gómez CE, Perdiguero B, Nájera JL, Sorzano CO, Jiménez V, González-Sanz R, Esteban M. Gómez CE, et al. J Virol. 2012 May;86(9):5026-38. doi: 10.1128/JVI.06684-11. Epub 2012 Mar 14. J Virol. 2012. PMID: 22419805 Free PMC article. - Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis.
Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ahmed M, et al. J Virol. 2003 Apr;77(8):4646-57. doi: 10.1128/jvi.77.8.4646-4657.2003. J Virol. 2003. PMID: 12663771 Free PMC article. - The highly virulent variola and monkeypox viruses express secreted inhibitors of type I interferon.
Fernández de Marco Mdel M, Alejo A, Hudson P, Damon IK, Alcami A. Fernández de Marco Mdel M, et al. FASEB J. 2010 May;24(5):1479-88. doi: 10.1096/fj.09-144733. Epub 2009 Dec 17. FASEB J. 2010. PMID: 20019241 Free PMC article. - Antiviral actions of interferons.
Samuel CE. Samuel CE. Clin Microbiol Rev. 2001 Oct;14(4):778-809, table of contents. doi: 10.1128/CMR.14.4.778-809.2001. Clin Microbiol Rev. 2001. PMID: 11585785 Free PMC article. Review.
References
- Alcamí A, Khanna A, Paul N L, Smith G L. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80:949–959. - PubMed
- Alcamí A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. - PubMed
- Beattie E, Tartaglia J, Paoletti E. Vaccinia-virus encoded eIF-2α homolog abrogates the antiviral effect of interferon. Virology. 1991;183:419–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources