MDM2 inhibits p300-mediated p53 acetylation and activation by forming a ternary complex with the two proteins - PubMed (original) (raw)
MDM2 inhibits p300-mediated p53 acetylation and activation by forming a ternary complex with the two proteins
E Kobet et al. Proc Natl Acad Sci U S A. 2000.
Abstract
p300 acetylates and activates the tumor suppressor p53 after DNA damage. Here, we show that MDM2, a negative-feedback regulator of p53, inhibited p300-mediated p53 acetylation by complexing with these two proteins. First, we purified a p300-MDM2-p53 protein complex from HeLa nuclear extracts, which was inactive in p53 acetylation, but active in histone acetylation. Also, wild-type, but not N-terminally deleted, MDM2 inhibited p53 acetylation by p300 in vitro and in vivo. This inhibition was specific for p53, because MDM2 did not affect acetylation of histones or the C terminus of p73 by p300. Consequently, wild-type, but not the mutant, MDM2 repressed the p300-stimulated sequence-specific DNA-binding and transcriptional activities of p53. These results demonstrate that an additional mechanism of p53 inactivation by MDM2 is to inhibit p53 acetylation by p300.
Figures
Figure 1
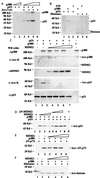
Association of MDM2 with p300 and p53 in the nucleus. (A) Purification of the MDM2-associated complex from HeLa nuclear extracts. The MDM2-associated protein complex was purified from human HeLa nuclear extracts through chromatography as described in_Materials and Methods_. Fractions (25 μl) from the last Superdex 200 column were analyzed on SDS/PAGE, followed by WB by using antibodies against p300 (Upper), MDM2, and p53, respectively, in this order. Molecular weight markers at the top of_A_ indicate the fractions where corresponding size markers coeluted. Fraction 18 was analyzed on SDS/PAGE and by silver staining, as shown in B.
Figure 2
Inhibition of p300-mediated p53 acetylation by MDM2 in vitro. (A) Establishment of p53 acetylation by p300 in vitro. Recombinant human p53 (50 ng) purified from bacteria and 250 nM acetyl CoA were used in the reaction as indicated; 100 ng (lanes 1 and 3), 200 ng (lanes 2 and 4), and 300 ng (lanes 5 and 6) of recombinant p300 purified from baculovirus-infected insect cells were used. Acetylated p53 was detected by WB by using antiacetylated Lys antibodies (Upstate Biotechnology). (B) Histone and p53 acetylation by recombinant p300 or the native p300 complex purified from HeLa nuclear extracts (fraction 18 of Fig. 1 A and B); 1 μl of14C-labeled acetyl CoA instead of nonlabeled acetyl CoA was used in each reaction. Histones (100 ng; Sigma), 50 ng of p53, 100 ng of p300, or 200 ng of the p300-containing fraction 18 from Superdex 200 were used in this experiment as indicated. Protein acetylation was detected by autoradiography. (C) MDM2 inhibits p53 acetylation by p300. In the acetylation reaction, 75 ng of p53, 200 ng of p300, and 250 nM acetyl CoA were used in this reaction as indicated; 200, 400, and 600 ng of recombinant MDM2 purified from baculovirus were used in lanes 3–5, respectively, and 600 ng of MDM2 for lane 6. Acetylated p53 was detected by antiacetylated Lys antibodies. (D) N-terminally deleted mutant MDM2 does not inhibit p53 acetylation by p300. The same acetylation reaction was performed as that in C; 200, 400, and 600 ng of either MDM2 or its N-terminal truncated mutant (ΔN-MDM2) were used as indicated. (E) MDM2 does not affect acetylation of the p73 C-terminal domain by p300; 75 ng of the p73 C-terminal fragment purified from bacteria (X.Z. and H.L., unpublished data) was used as a substrate. The same amounts of p300 and MDM2 as those in_C_ were used in this experiment. (F) MDM2 does not affect acetylation of histones by p300. The same assay was conducted as that in E except that 40 ng of histones (Sigma) was used in this assay.
Figure 3
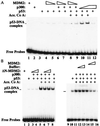
Wild-type but not the N-terminally deleted mutant MDM2 reduces enhancement of the sequence-specific DNA-binding activity of p53 by p300 in vitro. (A) Effect of MDM2 on sequence-specific DNA-binding activity of p53. In the EMSA experiment, 50 ng of p53, 250 nM acetyl-CoA, 100 ng (lane 10) or 200 ng (other lanes with +) of p300, and 200 ng (lanes 5, 7, and 9) or 400 ng (other lanes with +) of MDM2 were used as indicated on top. (B) Wild-type but not the N-terminally deleted mutant MDM2 inhibits the enhancement of DNA-binding activity by p300 of p53. The same EMSA reaction was carried out as that in A, except two controls were included here, the N-terminally truncated MDM2 mutant (200 or 400 ng as indicated on top) and the buffer (the same volumes as those for MDM2 proteins) used for preparation of MDM2 proteins.
Figure 4
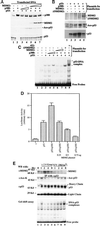
MDM2 inhibits p53 acetylation and activation by p300 in vivo. (A) MDM2 reduces p300-mediated p53 acetylation in cells. H1299 cells were transfected with plasmids encoding p53 (0.3 μg), p300 (0.5 μg), and/or MDM2 (0.5, 1, or 2 μg) as indicated on top. MG132 (5 μM) was added into media 12 h before harvesting. Cell lysates were prepared 36 h posttransfection for WB analysis; 400 μg of proteins were loaded onto a 10% SDS gel. (B) Wild-type but not N-terminally deleted MDM2 reduces p53 acetylation and level. The same transfection as that in A was conducted except the N-terminally deleted MDM2 (ΔNMDM2; 2 μg) was used without MG132 in this experiment. Asterisks indicate nonspecific signals. (C) MDM2 reduces the sequence-specific DNA-binding activity of p53 in cells. The exact same transfection as that in A was carried out. Nuclear extracts were prepared for EMSA analysis. Proteins (15 μg) were used for each reaction except lane 1; 1 μg of 421 was used in lane 9. (D) MDM2 reverses the enhancement of p53-dependent transcription by p300 in cells. As indicated, plasmids encoding no protein as a control (1 μg; C), p53 (50 ng) alone, or with p300 (0.15 μg) or with MDM2, and with a luciferase reporter gene (0.2 μg) driven by the p53RE motif derived from the MDM2 promoter (35), as well as a β-galactosidase reporter plasmid (0.1 μg) as an internal control, were introduced into H1299 cells (5 × 104 cells per 35 mm dish) by using Lipofectamine (GIBCO/BRL). Posttransfection (48 h) and 12 h after MG132 treatment (5 μM), cells were harvested for luciferase assays. Each column represents the mean data of three experiments. The bars denote that deviation of errors. (E) UV and γ irradiation differentially regulate p53 acetylation and DNA-binding activity, which are reciprocal to the MDM2 level. Tera-2 cells were irradiated with UV or γ ray, as indicated on top and harvested at different time points postirradiation for immunoprecipitation-WB. Proteins (300 μg) of the cell lysates from each time point were used for immunoprecipitation-WB by using antibodies as indicated (two middle panels). Nuclear extracts with 150 μg of proteins were directly loaded onto a 10% SDS gel for WB by using an anti-MDM2 antibody (Upper). EMSA (Lower) was carried out by using 15 μg of proteins in nuclear extracts and the 32P-labeled p53RE-containing DNA probes as described in Materials and Methods. αAce-K denotes the antibody specifically against the acetylated Lys. Similar results to that of E were also obtained by using F9 cells. All of these experiments were reproducible.
Similar articles
- MDM2 inhibits PCAF (p300/CREB-binding protein-associated factor)-mediated p53 acetylation.
Jin Y, Zeng SX, Dai MS, Yang XJ, Lu H. Jin Y, et al. J Biol Chem. 2002 Aug 23;277(34):30838-43. doi: 10.1074/jbc.M204078200. Epub 2002 Jun 14. J Biol Chem. 2002. PMID: 12068014 - MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation.
Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao TP. Ito A, et al. EMBO J. 2002 Nov 15;21(22):6236-45. doi: 10.1093/emboj/cdf616. EMBO J. 2002. PMID: 12426395 Free PMC article. - Defective p53 post-translational modification required for wild type p53 inactivation in malignant epithelial cells with mdm2 gene amplification.
Knights CD, Liu Y, Appella E, Kulesz-Martin M. Knights CD, et al. J Biol Chem. 2003 Dec 26;278(52):52890-900. doi: 10.1074/jbc.M300279200. Epub 2003 Oct 10. J Biol Chem. 2003. PMID: 14555661 - To die or not to die: a HAT trick.
Tyteca S, Legube G, Trouche D. Tyteca S, et al. Mol Cell. 2006 Dec 28;24(6):807-8. doi: 10.1016/j.molcel.2006.12.005. Mol Cell. 2006. PMID: 17189182 Review. - Dynamics of the p53 acetylation pathway.
Gu W, Luo J, Brooks CL, Nikolaev AY, Li M. Gu W, et al. Novartis Found Symp. 2004;259:197-205; discussion 205-7, 223-5. Novartis Found Symp. 2004. PMID: 15171255 Review.
Cited by
- Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2.
Ferreon JC, Lee CW, Arai M, Martinez-Yamout MA, Dyson HJ, Wright PE. Ferreon JC, et al. Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6591-6. doi: 10.1073/pnas.0811023106. Epub 2009 Apr 8. Proc Natl Acad Sci U S A. 2009. PMID: 19357310 Free PMC article. - Targeting p53-MDM2-MDMX loop for cancer therapy.
Zhang Q, Zeng SX, Lu H. Zhang Q, et al. Subcell Biochem. 2014;85:281-319. doi: 10.1007/978-94-017-9211-0_16. Subcell Biochem. 2014. PMID: 25201201 Free PMC article. Review. - Etoposide induces cell death via mitochondrial-dependent actions of p53.
Jamil S, Lam I, Majd M, Tsai SH, Duronio V. Jamil S, et al. Cancer Cell Int. 2015 Aug 7;15:79. doi: 10.1186/s12935-015-0231-z. eCollection 2015. Cancer Cell Int. 2015. PMID: 26251638 Free PMC article. - Hepatitis C virus impairs p53 via persistent overexpression of 3beta-hydroxysterol Delta24-reductase.
Nishimura T, Kohara M, Izumi K, Kasama Y, Hirata Y, Huang Y, Shuda M, Mukaidani C, Takano T, Tokunaga Y, Nuriya H, Satoh M, Saito M, Kai C, Tsukiyama-Kohara K. Nishimura T, et al. J Biol Chem. 2009 Dec 25;284(52):36442-36452. doi: 10.1074/jbc.M109.043232. Epub 2009 Oct 27. J Biol Chem. 2009. PMID: 19861417 Free PMC article. - Mdm2 enhances ligase activity of parkin and facilitates mitophagy.
Kook S, Zhan X, Thibeault K, Ahmed MR, Gurevich VV, Gurevich EV. Kook S, et al. Sci Rep. 2020 Mar 19;10(1):5028. doi: 10.1038/s41598-020-61796-4. Sci Rep. 2020. PMID: 32193420 Free PMC article.
References
- Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. - PubMed
- Levine A J. Cell. 1997;88:323–331. - PubMed
- Giaccia A J, Kastan M B. Genes Dev. 1998;12:2973–2983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous