The yeast nucleoporin Nup53p specifically interacts with Nic96p and is directly involved in nuclear protein import - PubMed (original) (raw)
The yeast nucleoporin Nup53p specifically interacts with Nic96p and is directly involved in nuclear protein import
B Fahrenkrog et al. Mol Biol Cell. 2000 Nov.
Free PMC article
Abstract
The bidirectional nucleocytoplasmic transport of macromolecules is mediated by the nuclear pore complex (NPC) which, in yeast, is composed of approximately 30 different proteins (nucleoporins). Pre-embedding immunogold-electron microscopy revealed that Nic96p, an essential yeast nucleoporin, is located about the cytoplasmic and the nuclear periphery of the central channel, and near or at the distal ring of the yeast NPC. Genetic approaches further implicated Nic96p in nuclear protein import. To more specifically explore the potential role of Nic96p in nuclear protein import, we performed a two-hybrid screen with NIC96 as the bait against a yeast genomic library to identify transport factors and/or nucleoporins involved in nuclear protein import interacting with Nic96p. By doing so, we identified the yeast nucleoporin Nup53p, which also exhibits multiple locations within the yeast NPC and colocalizes with Nic96p in all its locations. Whereas Nup53p is directly involved in NLS-mediated protein import by its interaction with the yeast nuclear import receptor Kap95p, it appears not to participate in NES-dependent nuclear export.
Figures
Figure 1
Immunogold-EM localization of Nup53p in ProtA-Nup53p cells (i.e., Δ_nup53_[ProtA-Nup53p] strain; Table 1). (A) Triton X-100-extracted spheroplasts were preimmunolabeled with a polyclonal anti-protein A antibody directly conjugated to 8-nm colloidal gold. Shown are selected examples of gold-labeled NPCs in cross-sections along the NE. The antibody labeled the cytoplasmic (top) and the nuclear (middle) periphery of the central framework, and the nuclear basket (bottom). c, cytoplasm; n, nucleus. (B) Quantitative analysis of the gold particle distribution associated with the NPCs in the Δ_nup53_(ProtA-Nup53p) strain. For quantitative analysis 94 gold particles were scored. (C) Schematic representation of the close colocalization of Nic96p and Nup53p within the yeast NPC by their respective “location clouds” (Fahrenkrog_et al._, 2000). Accordingly, Nic96p is located about the cytoplasmic and the nuclear periphery of the central channel, and near or at the distal ring of the nuclear basket. Similarly, Nup53p resides on the cytoplasmic and the nuclear face of the central framework (i.e., close to or at the base of the cytoplasmic and the nuclear fibrils), and near the distal end of the nuclear basket fibrils. Scale bar, 100 nm (A).
Figure 2
Affinity purification of ProtA-TEV-Nup53p from Δ_nup53_(ProtA-TEV-Nup53p) cells (Table 1) reveals interaction with Nic96p. ProtA-TEV-Nup53p was affinity-purified by IgG-Sepharose chromatography and Nup53p was released by TEV-mediated proteolytic cleavage. Western blot analysis with a polyclonal anti-Nic96p antibody reveals specific interaction of Nup53p with Nic96p. Lane 1, NPC-containing fraction of a wild-type control strain (BMA41); lane 2, eluate derived from the wild-type control strain; lane 3, NPC-containing fraction of the strain expressing ProtA-TEV-Nup53p; and lane 4, eluate derived from the ProtA-TEV-Nup53p.
Figure 3
Thin-section electron micrographs of Δ_nup53_ cells (B) and of a wild-type BMA41 control strain (A) (for the cell strains see Table 1). Cells were grown at 30°C, fixed, embedded**,** and processed for thin-section electron microscopy. The morphology of the nucleus, the NE, and the NPCs in the Δ_nup53_ cells appears indistinguishable from that of the wild-type cells. Arrows mark the nuclear basket of the NPCs. Scale bars, 100 nm (A and B).
Figure 4
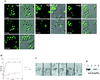
Nup53p mediates nuclear import of a NLS-GFP reporter. (A) Intracellular localization of the NLS-GFP reporter in Δ_nup53_, wild-type BMA41 control cells, and_nup49-313_ control cells (Table 1) after azide and deoxyglucose treatment (i.e., 0 min) and after recovery from the drug treatment in a glucose-containing medium (i.e., 15 and 120 min). Shown are confocal fluorescence micrographs (left) and coincident fluorescence/differential interference contrast images (right). (B) Quantification of the relative import rates in the Δ_nup53_ and the wild-type BMA41 control cells by counting cells harboring nuclear fluorescence as a function of time. (C) Immunogold-EM reveals that the NLS-GFP reporter accumulates within the nuclear basket of the yeast NPCs in the Δ_nup53_ cells (for quantification see Table 2). (D) Nup53p specifically interacts with the yeast nuclear import receptor Kap95p. For this purpose, ProtA-TEV-Nup53p was affinity purified by IgG-Sepharose chromatography and analyzed for copurifying components. Shown is a Western blot analysis with a polyclonal anti-Ka95p antibody revealing the specific interaction of Nup53p with Kap95p. Lane 1, NPC-containing fraction of a wild-type control strain (BMA41); lane 2, eluate derived from the wild-type control strain; lane 3, NPC-containing fraction of the strain expressing ProtA-TEV-Nup53p; and lane 4, eluate derived from the ProtA-TEV-Nup53p. Scale bar, 100 nm (C).
Figure 5
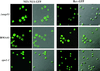
NES-mediated protein export monitored in Δ_nup53_ cells and in a wild-type BMA41 control strain (Table 1). An NLS-NES-GFP or a Rev-GFP fusion construct was expressed in Δ_nup53_ or BMA41 cells. The localization of the GFP reporter was analyzed after growing the cells at 30°C revealing accumulation of the GFP signal in the cytoplasm, thus indicating that the presence of Nup53p is not necessary for NES-mediated protein export. Shown are confocal fluorescence and coincident fluorescence/differential interference contrast images to localize the GFP reporters relative to the cell periphery.
Figure 6
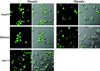
Yap1p-GFP export is not impaired in a Δ_nup53_ background. Yeast strains BMA41, Δ_nup53_, or xpo1-1 (Table 1) were transformed with pLDB419(Yap1-GFP), grown in selective medium in the presence or absence of the oxidant diamide. GFP fusion proteins are visualized by confocal fluorescence and coincident fluorescence/differential interference contrast optics.
Similar articles
- Nup2p, a yeast nucleoporin, functions in bidirectional transport of importin alpha.
Solsbacher J, Maurer P, Vogel F, Schlenstedt G. Solsbacher J, et al. Mol Cell Biol. 2000 Nov;20(22):8468-79. doi: 10.1128/MCB.20.22.8468-8479.2000. Mol Cell Biol. 2000. PMID: 11046143 Free PMC article. - Specific binding of the karyopherin Kap121p to a subunit of the nuclear pore complex containing Nup53p, Nup59p, and Nup170p.
Marelli M, Aitchison JD, Wozniak RW. Marelli M, et al. J Cell Biol. 1998 Dec 28;143(7):1813-30. doi: 10.1083/jcb.143.7.1813. J Cell Biol. 1998. PMID: 9864357 Free PMC article. - Molecular mechanisms of nuclear protein transport.
Moroianu J. Moroianu J. Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review. - Distinct nuclear import and export pathways mediated by members of the karyopherin beta family.
Moroianu J. Moroianu J. J Cell Biochem. 1998 Aug 1;70(2):231-9. J Cell Biochem. 1998. PMID: 9671229 Review.
Cited by
- Systematic Protein-Protein Interaction Analysis Reveals Intersubcomplex Contacts in the Nuclear Pore Complex.
Apelt L, Knockenhauer KE, Leksa NC, Benlasfer N, Schwartz TU, Stelzl U. Apelt L, et al. Mol Cell Proteomics. 2016 Aug;15(8):2594-606. doi: 10.1074/mcp.M115.054627. Epub 2016 May 18. Mol Cell Proteomics. 2016. PMID: 27194810 Free PMC article. - A subset of FG-nucleoporins is necessary for efficient Msn5-mediated nuclear protein export.
Finn EM, DeRoo EP, Clement GW, Rao S, Kruse SE, Kokanovich KM, Belanger KD. Finn EM, et al. Biochim Biophys Acta. 2013 May;1833(5):1096-103. doi: 10.1016/j.bbamcr.2012.12.020. Epub 2013 Jan 4. Biochim Biophys Acta. 2013. PMID: 23295456 Free PMC article. - The nuclear localization of SWI/SNF proteins is subjected to oxygen regulation.
Dastidar RG, Hooda J, Shah A, Cao TM, Henke RM, Zhang L. Dastidar RG, et al. Cell Biosci. 2012 Aug 29;2(1):30. doi: 10.1186/2045-3701-2-30. Cell Biosci. 2012. PMID: 22932476 Free PMC article. - The yeast nuclear pore complex and transport through it.
Aitchison JD, Rout MP. Aitchison JD, et al. Genetics. 2012 Mar;190(3):855-83. doi: 10.1534/genetics.111.127803. Genetics. 2012. PMID: 22419078 Free PMC article. - A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope.
Marelli M, Lusk CP, Chan H, Aitchison JD, Wozniak RW. Marelli M, et al. J Cell Biol. 2001 May 14;153(4):709-24. doi: 10.1083/jcb.153.4.709. J Cell Biol. 2001. PMID: 11352933 Free PMC article.
References
- Baudin-Baillieu A, Guillemet E, Cullin C, Lacroute F. Construction of a yeast strain deleted for the TRP1 promotor and coding region that enhances the efficiency of the polymerase chain reaction-disruption method. Yeast. 1997;13:353–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases